Translate this page into:
Study of effectiveness of methotrexate oral pulse in refractory chronic urticaria
*Corresponding author: Arun Kumar Yadav, Department of Dermatology, Baba Raghav Das Medical College, Gorakhpur, Uttar Pradesh, India. rahuldreams1992@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yadav AK. Study of effectiveness of methotrexate oral pulse in refractory chronic urticaria. Indian J Skin Allergy. 2024;3:66-70. doi: 10.25259/IJSA_28_2023
Abstract
Objectives:
Chronic urticaria (CU) is an unexplained problem that needs to be addressed, and since patients also experience the morbidity associated with irritable itch, they must take a significant quantum of antihistamines. Autoantibodies in the blood cause recurrent flare-ups in autoreactive urticaria when the symptoms are more noticeable. As a result, the need for an adjuvant drug to lessen the weight of pills is felt. The aim of this study was to find out the effectiveness of oral methotrexate (MTX) pulse with antihistamines in chronic refractory urticaria.
Material and Methods:
This was the present longitudinal intervention. The study lasted six months and was carried out at the Department of Dermatology in a hospital with tertiary care status. Fifty patients of chronic spontaneous urticaria have been selected by simple random sampling. All subjects have been given Oral Methotrexate, 15 mg once weekly along with folic acid. Besides, MTX all cases have been given oral desloratadine 5 mg twice daily.
Results:
Most patients have seen a significant decline in urticaria activity score 7 (UAS7) and dermatological life quality index. The baseline mean UAS7 reduced significantly from 30.16 ± 8.65 to 1.24 ± 2.24 with a statistically significant P < 0.01. No serious side effects were seen except mildly raised liver transaminases in seven patients.
Conclusion:
When standard second-generation antihistamines are insufficient at treating chronic uncontrolled urticaria, MTX is a very safe, well-tolerated, and economical treatment option.
Keywords
Antihistamine
Methotrexate
Urticaria activity score
Chronic urticaria
Dermatological life quality index (DLQI)
INTRODUCTION
Transient cutaneous or mucosal bumps due to plasma leaking are defining urticaria. Wheals are small, superficial dermal swellings, and angioedema refers to larger, deeper skin or mucosal swellings.[1] While angioedema is very unpleasant, less clearly defined, and does not change color, welts are generally itchy and pink or pale in the center. It has an enormous impact on the patient’s everyday activities and quality of life.[2,3] Urticaria has a reported 7.8–22.3% lifelong prevalence, with a point prevalence of nearly 1%.[4,5] A person’s chance to experience urticaria over their lifetime ranges between 15% and 20%.[6] The illness generally lasts for 1–5 years.[3]
Chronic urticaria (CU) is primarily diagnosed clinically. Thus, only a few tests backed up by a complete clinical history and examination are required.[7] The standard and recommended treatment grounded on excellent safety and tolerability profiles are second-generation non-sedating antihistamines (such as levocetirizine, desloratadine, and fexofenadine). Antihistamines can constantly be used in clinical practice at up to 2–4-fold larger doses than advised for treating problematic or inadequately controlled urticaria.[8-10] Corticosteroids, intravenous immunoglobulin, omalizumab, tacrolimus, methotrexate (MTX), cyclosporine, MTX, mycophenolate mofetil, cyclophosphamide, and narrow-band ultraviolet B are other therapeutic approaches for difficult or resistant urticaria.[11-14]
It has been debated that MTX is useful in treating chronic urticaria of the idiopathic, autoimmune, and steroid-dependent kinds.[15,16] The intention of the present study is to determine the effectiveness of MTX oral pulse in treating chronic refractory urticaria.
MATERIAL AND METHODS
The current longitudinal intervention research was carried out in a tertiary care facility’s dermatology department for six months. Permission was taken from the Institutional Ethical Board before the commencement of the study. The patient’s age falls between 18 and 60 years. All patients belonged to the same geographical area and had more or less similar demographic features. Most of the patients fall into the lower and lower middle classes of socioeconomic status. Patient consent was obtained and they were selected based on the following inclusion and exclusion criteria.
Inclusion criteria
The following criteria were included in the study:
Patients with chronic urticaria not controlled to even four-fold normal doses of second-generation antihistamines (SgAH) for three months, especially levocetirizine, bilastine, and olopatadine
Patients whose total disease duration was more than six months
Patients without any comorbid conditions such as diabetes, hypertension, and any liver or kidney impairment.
Exclusion criteria
The following criteria were excluded from the study:
Patients of acute urticarial
Patients controlled on up-dosing of SgAH (up to four times normal doses)
Patients with liver or any other organ function impairment
Pregnant or lactating female patients
Pediatric patients.
Simple random sampling was done. Fifty patients of chronic refractory urticaria were diagnosed clinically enrolled for the study. All the patients have been given oral MTX, 15 mg once weekly, along with folic acid. Besides MTX, loratadine 5 mg twice daily was given to each patient for six months as shown in Figure 1. Following a month of continuous 15 mg weekly administration, the MTX dose was gradually lowered at a pace of 2.5 mg/month, and at the six-month mark, it was stopped at 2.5 mg/week. The cost of the treatment under study was covered by the patient itself. Assessment of baseline urticaria activity score 7 (UAS7) and dermatological life quality index (DLQI) has been done in all patients, which were compared with post-treatment scores. Urticaria-free = 0, good-controlled urticaria = 1–6, mild urticaria = 7–15, moderate activity urticaria = 16–27, and severe activity urticaria = 28–42 were the four UAS7 score-based health conditions [Table 1].[17]
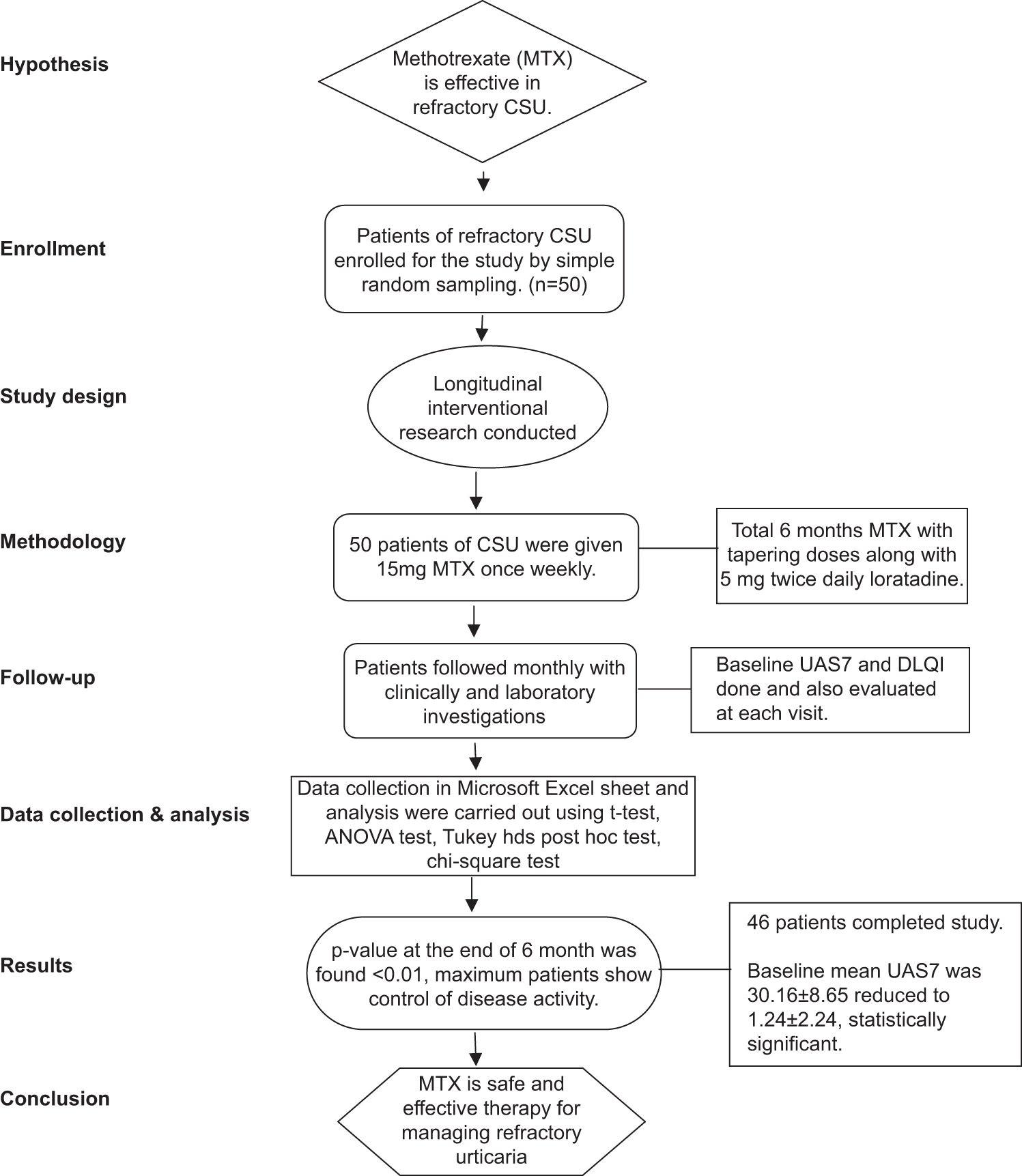
- Study Flow Chart; CSU: Chronic spontaneous urticaria, ANOVA: Analysis of Variance, UAS7: Urticaria activity score 7, DLQI: Dermatological life quality index.
| Score | Wheals | Pruritus |
|---|---|---|
| 0 | None | None |
| 1 | Mild (1-20 wheals every 24 h) | Mild (present but not bothersome or obtrusive) |
| 2 | Moderate (20-50 wheals every 24 h) | Moderate (difficult but does not disrupt sleep or daily activities) |
| 3 | Severe (more than 50 wheals/24 h or big patches of confluent wheals) | Severe (severe pruritus so unpleasant that it prevents one from going about one's regular duties or sleeping) |
UAS7: Urticaria activity score 7, CSU: Chronic spontaneous urticaria.
Total score: 0–6 for each day is calculated over one week and added (maximum 42).
Dermatology life quality index (DLQI) is a set of fixed questionnaires having 10 questions. The maximum score for each question was three, and the minimum score was 0.[18] It is used to determine the quality of life in various dermatological conditions,including urticaria. To give the DLQI scores context, a number of tested “band adjectives” were established in 2005.[19] Following are these bands: 0–1 indicates no influence on the patient’s life; 2–5 is a minor influence; 6–10 is a moderate influence; 11–20 is a very substantial influence; and 30 is an incredibly significant influence.
Baseline routine investigations such as general blood picture, liver function test (LFT), kidney function test (KFT), and urine pregnancy test in females to rule out pregnancy were done before starting treatment. Married female patients were advised to refrain from conception until treatment continued. Patients are followed monthly, along with necessary laboratory investigations such as complete blood count, LFT, and KFT to monitor any MTX -induced adverse event. Each scheduled visit also comprised a thorough history, the determination of UAS7, and a questionnaire-based assessment of each patient’s quality of life. After completion of the study, patients were followed for the next three months to monitor the post-treatment disease activity.
All the data gathered were stored in a Microsoft Excel sheet, and utilizing IBM Statistical Package for the Social Science Statistics for Windows, version 23 (IBM Crop., Armonk, NY), both descriptive and inferential statistics were carried out using t-test, analysis of variance test, Tukey HSD post hoc test, and Chi-square test. Results were considered significant if P < 0.05.
RESULTS
Four patients dropped out of the study while 46 had completed it. The mean age of patients was calculated and found to be 32.6 ± 9.68 [Table 2]. A greater number of subjects were female as compared to males (males = 24 and females = 26) [Table 3]. All patients had their baseline DLQI compared, and it was discovered that the findings were statistically significant with P < 0.01 when comparing the baseline and six-month mean scores. The baseline DLQI of the study group was 18 ± 5.52 and in six months, it reduced to 1.48 ± 1.73 [Table 4] that is statistically significant. When the UAS7 scores at the beginning, 1 month, 3 months, and 6 months were compared for all patients, it was discovered that the outcomes were statistically significant with P < 0.01 [Table 5].
| Mean age | Standard deviation |
|---|---|
| 32.66 | 9.68 |
| Male | Female | ||
|---|---|---|---|
| n | % | n | % |
| 24 | 48 | 26 | 52 |
| DLQI | t-test | P-value | |||
|---|---|---|---|---|---|
| Baseline | six months | ||||
| Mean | SD | Mean | SD | ||
| 18.74 | 5.52 | 1.48 | 1.73 | 24.58 | <0.01* |
| UAS7 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Before treatment | 1 month | 3 months | 6 months | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 30.16 | 8.65 | 12.60 | 5.82 | 4.63 | 3.39 | 1.24 | 2.24 | <0.01* |
The baseline average UAS7 was 30.16 ± 8.65 and plummeted to 1.24 ± 2.24 over a six-month period. A comparison of subjects’ UAS grading was done and found that a maximum of subjects, 50% were urticaria free while 34% were well controlled at the end of treatment. This indicates that 84% of patients were able to adequately manage their disease activity. Only four patients (8%) fell into the mild activity urticaria category (UAS7) and had not been able to control their symptoms [Table 6]. The safety of MTX was monitored clinically and with a laboratory investigation at each monthly scheduled visit. No serious adverse effects were seen in any patient except mildly raised liver transaminases in seven patients, which do not lead to discontinuation of therapy and normalize with tapering of MTX dose. Subjects were monitored for the following three months after the trial was over, and no patient had worsening disease activity.
| UAS7 | |||||||
|---|---|---|---|---|---|---|---|
| Well controlled (UAS7=1–6) | Urticaria free (UAS7=0) | Mild activity (UAS7=7–15) | Moderate activity (UAS7=16–27) | ||||
| n | % | n | % | n | % | n | % |
| 17 | 34 | 25 | 50 | 4 | 8 | 0 | 0 |
UAS7: Urticaria activity score 7.
DISCUSSION
Chronic idiopathic urticaria, known for a very long time, is a very troublesome ailment that can always interfere with a person’s social, professional, and personal life. This shows up as pruritic, elevated, varying-sized wheals with serpiginous edges, and blanched centers all over the body that might occasionally merge.[20] For a period of more than six weeks, it may emerge every day or on the majority of days. In addition to addressing and avoiding the catalyst causes, the current recommendation is to make all safe attempts to completely suppress urticaria symptoms. The treatment remains a challenge for clinicians. At present, a methodical approach is recommended.[21] First-line therapy comprises a non-sedating H1 antihistamine at standard doses. The purpose of the current research was to evaluate the efficacy of MTX pulse therapy, along with antihistamine, in chronic refractory urticaria.
The study was conducted at the Department of Dermatology in a tertiary care hospital among 50 patients. All the subjects have been given oral MTX, 15 mg once weekly along with folic acid. Loratadine 5 mg twice daily is also given for six months. No control group was taken to keep the study simple and more compliant for the subjects.
The maximum patients were under the age group of 30– 40 years, and females were more in number as compared to males suggesting a higher disease incidence.
In our study, a considerable reduction in urticaria severity score, from a baseline mean of 30.16–1.24 with P < 0.01. In a comparable manner, in a study by Sagi et al. using MTX, it was observed that seven of the eight patients with CU who were treated with weekly doses of 15–25 mg of MTX showed complete remission, with the onset of effect seen at 3–5 weeks and maintained for 2–15 months. These patients had previously responded poorly to oral steroids, antihistamines, and other immunosuppressants.[22] Similarly, research by Perez et al. revealed that MTX, given in doses ranging from 5 to 15 mg/week, significantly improved the symptoms in 16 patients with steroid-dependent chronic urticaria, including ten cases of chronic urticaria, four cases of urticarial vasculitis, and two cases of angioedema [Table 7].[23] Out of 16 patients 12 responded to the treatment; two were able to stop taking steroids, and seven were able to gradually reduce their oral steroid dosage.
| Study | Method | Results |
|---|---|---|
| Godse[9] | Sample size=4 patients of refractory CSU MTX 2.5 mg twice daily for 2 consecutive days per week. | All 4 showed good control of disease activity. |
| Sagi et al.[22] | Sample size=8 (males-2, females-6) MTX-15 mg/week | A complete response was achieved in seven out of eight patients (87%). |
| Perez et al.[23] | Sample size=16 patients of steroid-dependent CSU. MTX 10–15 mg/week (total cumulative dose range 15–600 mg, average 135 mg) |
Seven patients significantly improved, three patients exhibited moderate improvement, and two patients cleared. Non Responders showed autoantibodies. |
CSU: Chronic spontaneous urticaria, MTX: Methotrexate.
A comparison of the DLQI was carried out in all subjects,and it was found that while comparing the mean score of baseline and six months. Noteworthy, results were obtained with P-value of lower than 0.01. This shows that the quality of life experienced by patients was poor before receiving the treatment, but after receiving the treatment, around 50–85% of the participants exhibited a substantial improvement in their scores. Larger, more robust investigations are needed, nonetheless, to firmly establish this assertion. In line with the ambiguous findings of our long-term intervention study, it could be beneficial to look at MTX and other immunosuppressants in more detail and compare how they affect chronic refractory urticaria.[24]
One of our study’s shortcomings was the brief treatment period. Larger placebo-controlled randomized trials are required to further demonstrate the efficacy of therapy in chronic uncontrolled urticaria because the body of literature now available demonstrates that it has a positive effect on other types of chronic urticaria.
CONCLUSION
From this study, it can be postulated that MTX is a very safe, well-tolerated, and effective therapy to manage chronic spontaneous urticaria not responding to higher doses of SgAH.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
Patient’s consent was not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship
Nil.
References
- Urticaria In: Burns DA, Breathnach SM, Cox NH, eds. Rooks textbook of dermatology (8th ed). Hoboken: Blackwell Publishing Ltd; 2010. p. :22.1-22.39.
- [Google Scholar]
- The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136:197-201.
- [CrossRef] [PubMed] [Google Scholar]
- Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317-30.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14:214-20.
- [Google Scholar]
- Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174:996-1004.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of a history-based diagnostic approach in chronic urticaria and angioedema. Arch Dermatol. 1998;134:1575-80.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: A randomized, placebo-controlled, crossover study. J Allergy Clin Immunol. 2009;123:672-9.
- [CrossRef] [PubMed] [Google Scholar]
- Updosing of antihistamines to improve control of chronic urticaria. Indian J Dermatol Venereol Leprol. 2010;76:61-2.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing the dose of cetirizine may lead to better control of chronic idiopathic urticaria: An open study of 21 patients. Br J Dermatol. 2007;157:803-4.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized placebo-controlled trial comparing desloratadine and montelukast in monotherapy and desloratadine plus montelukast in combined therapy for chronic idiopathic urticaria. J Allergy Clin Immunol. 2004;114:619-25.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of a short course of oral prednisone in antihistamine-resistant chronic urticaria: A retrospective analysis. J Investing Allergol Clin Immunol. 2010;20:386-90.
- [Google Scholar]
- Cyclosporine in chronic idiopathic urticaria: A double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol. 2006;55:705-9.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate-responsive chronic idiopathic urticaria: A report of two cases. Br J Dermatol. 2001;145:340-3.
- [CrossRef] [PubMed] [Google Scholar]
- The use of mycophenolate mofetil for the treatment of autoimmune and chronic idiopathic urticaria: Experience in 19 patients. J Am Acad Dermatol. 2012;66:767-70.
- [CrossRef] [PubMed] [Google Scholar]
- Oral cyclophosphamide in a case of cyclosporin and steroid-resistant chronic urticaria showing autoreactivity on autologous serum skin testing. Clin Exp Dermatol. 2005;30:582-3.
- [CrossRef] [PubMed] [Google Scholar]
- The EAACI/GA(2) LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy. 2014;69:868-87.
- [CrossRef] [PubMed] [Google Scholar]
- Confirmation of the dermatology life quality index as an outgrowth measure for urticaria-related quality of life. Ann Allergy Asthma Immunol. 2004;931:42-6.
- [Google Scholar]
- Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol. 2005;125:659-64.
- [CrossRef] [PubMed] [Google Scholar]
- Harrison's principle of internal medicine New York: McGraw Hill Professional; 2012.
- [Google Scholar]
- EAACI/GA(2)LEN task force consensus report: The autologous serum skin test in urticaria. Allergy. 2009;64:1256-68.
- [CrossRef] [PubMed] [Google Scholar]
- Substitution for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate: A useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4.
- [CrossRef] [PubMed] [Google Scholar]
- Double dose of cetirizine hydrochloride is effective for patients with urticaria resistant: A prospective, randomized, non-blinded, comparative clinical study and assessment of quality of life. J Dermatolog Treat. 2013;24:153-60.
- [CrossRef] [PubMed] [Google Scholar]






