Translate this page into:
The effectiveness of bilastine and fexofenadine updosing in the management of chronic spontaneous urticaria
-
Received: ,
Accepted: ,
How to cite this article: Shah B, Choudhary A, Mistry D, Jangid N, Shah S, Kamat S, et al. The effectiveness of bilastine and fexofenadine updosing in the management of chronic spontaneous urticaria. Indian J Skin Allergy 2022;1:40-5.
Abstract
Objectives:
In patients not responding to standard dosages of second-generation antihistamines (SGAHs), updosing up to 4-fold is recommended. Even though SGAHs are safe, the concerns regarding their safety after updosing still remain and the studies directly comparing the updosing of different SGAHs are lacking thus making the choice of drug difficult.
Material and Methods:
Eighty patients with chronic spontaneous urticaria (CSU) were randomized to receive either bilastine or fexofenadine (40 in each group). Patients were started on conventional dose of either drug (bilastine 20 mg or fexofenadine 180 mg) for the first 2 weeks. Those patients who remained symptomatic after 2 weeks (UAS ≥ 7) were given double dose of bilastine (20 mg BD) or fexofenadine (180 mg BD), respectively, for another 2 weeks. Control of urticaria was assessed by evaluating UAS score. Patients’ quality of life was assessed by CU-Q2oL questionnaire. Safety was evaluated by analyzing the adverse events (AEs) reported by the patients.
Results:
Seventy-four patients completed the study. Forty-one patients (bilastine – 23 and fexofenadine – 18) achieved adequate control of urticaria (UAS ≤ 6) at week 2. Out of 33 patients who were given high dose of bilastine or fexofenadine, 17 patients (bilastine – 9 and fexofenadine – 8) achieved adequate control of urticaria at week 4. Bilastine was associated with significant improvement in quality of life of patients compared to fexofenadine (32.38 ± 5.83 vs. 38.71 ± 5.92.; P < 0.005). Thirteen patients (six in bilastine group and seven in fexofenadine group) reported one or more AEs with sedation being the most common side effect.
Conclusion:
Updosing of bilastine provided relief from urticaria symptoms, improved quality of life in the majority of the patients without compromising somnolence or safety. Bilastine was associated with improved quality of life and less sedation compared to fexofenadine.
Keywords
Bilastine
Fexofenadine
Updosing
Chronic spontaneous urticaria
Quality of life
INTRODUCTION
Chronic urticaria (CU) is defined by the occurrence of wheals (hives), angioedema, or both for more than 6 weeks.[1] It is common in every country globally, and its prevalence has increased 2–10-fold over the past decade.[2] Maurer et al. in their systematic review and meta-analysis reported an overall lifetime CU prevalence of 4.4%.[3] CU has a significant impact on health-related quality of life (HRQoL), similar or greater than moderate-to-severe psoriasis, atopic dermatitis, asthma, and severe coronary artery disease requiring bypass grafting.[2] As a consequence, effective therapy is of paramount importance.
Since histamine plays a central role in pathogenesis of urticaria, EAACI/GA2LEN/EDF/WAO and Indian guidelines recommend second-generation antihistamines (SGAHs) as first-line therapy in the management of CU.[1,4] However, on average, only 50% of patients have adequate response to standard doses of SGAHs because histamine may reach very high concentrations in the skin, due to its poor diffusibility in the dermis.[5,6] Guillén-Aguinaga et al. in their systematic review reported that 60% of patients were unresponsive to standard dose antihistamines.[7] Various studies reported similar results where the percentage of patients having adequate response to standard dosage of antihistamines was in the range of 18–44%.[8-10]
In such patients who do not responds to standard dosages of SGAH, guidelines recommend updosing of antihistamines up to 4-fold.[1,4] Numerous clinical studies support the clinical benefit of higher dosage of antihistamines.[4] Even though SGAHs are safe with much reduced brain penetration, the concerns regarding the safety after updosing still remain with SGAHs.[6] In a study of patients’ perspective of effectiveness and side effects of H1-antihistamine updosing, Weller et al. reported that out of 319 patients, >20% of patients raised concerns regarding possible side effects and long-term harmful effects of SGAHs. Many patients in the study stated that unwanted effects were worse than with the standard dose.[11]
Based on above facts, two major factors are very important while using higher dosages of SGAHs. First is freedom from unwanted side effects, particularly sedation, so that updosing may occur safely. Second is strong clinical efficacy, preferably with a rapid onset and long duration of action.
Bilastine is a novel SGAH with faster and longer duration of action, lowest H1-receptor occupancy (H1RO) in the CNS compared to other SGAHs.[12] In multiple randomized clinical and real-world studies, bilastine was associated with significant improvement in signs and symptoms of urticaria in standard as well as in higher dosage.[13-18]
However, the studies directly comparing the effectiveness and safety of bilastine updosing to other SGAH are lacking especially in Indian setup. Thus, we conducted this study with an objective to compare the effectiveness and safety of bilastine updosing with fexofenadine updosing in patients with chronic spontaneous urticaria (CSU).
MATERIAL AND METHODS
Study design
This was a randomized, comparative, open-label, two-arm, and single-center study. Participants were recruited from April 2020 to October 2020 and followed up for 4 weeks and all patients provided informed written consent. The study was approved by the Institutional Ethical Committee.
Study participants
This study was done in tertiary care hospital in Ahmedabad. Patients within the age group of 18–60 years and with a clinical history of CSU for at least 6 weeks during the past 3 months without an identifiable cause and urticaria activity score 7 (UAS7) of ≥7 were recruited.
Patients with physical urticaria, drug-induced urticaria, urticarial vasculitis; patients with any other dermatological condition associated with pruritus; pregnant or nursing females; patients with immunosuppressive disease or on immunosuppressive drugs; and patients with evidence of cardiac, respiratory, gastrointestinal, and renal disease were excluded from the study.
Patient follow-up
At baseline visit, clinical evaluation of patients was done to assess urticaria activity during the preceding week based on UAS7. Patients’ quality of life was assessed using CU-Q2oL questionnaire. Patients were randomized to receive either bilastine 20 mg once daily or fexofenadine 180 mg once daily for a period of 2 weeks. At this visit, patients were given diary to note down UAS (from 0, no itch and no wheals, to 3, itch at its worst with multiple wheals) and adverse events (AEs). Patients were followed at week 2 and week 4 [Figure 1].

- Patient selection criteria and treatment randomization.
During the second visit at week 2, patients were again evaluated for their USA7 and CU-Q2oL. Patients were further evaluated regarding urticaria associated discomfort on visual analogue scale (VAS). At this visit, patients with a UAS7 score of 6 and less were considered responsive to drug and were instructed to continue the same treatment. These patients were not considered for further analysis.
The remaining patients with USA7 score of 7 and above were given a double dose of bilastine (20 mg twice a day) and fexofenadine (180 mg twice a day). At visit 3, all evaluations were repeated and patients were asked to report the AEs experience by them during previous 2 weeks. Data were collected using standardized case report forms at baseline; day 14; and day 28.
Study assessment
The primary effectiveness end point was proportion of patients becoming symptom free at weeks 2 and 4. The secondary end points were mean reduction in UAS7 score at week 2 and 3, improvement in quality of life of patients based on CU-Q2oL at weeks 2 and 4, patients’ satisfaction with treatment at weeks 2 and 4, and sedation potential of treatment at weeks 2 and 4.
The safety of each treatment regime was analyzed by assessing the proportion of patients showing one or more AE during the study period.
Statistical analysis
Results were presented as mean scores and groups are compared using one-way ANOVA with Tukey HSD test and Fisher’s exact test. Data were analyzed using the SPSS software version 2.
RESULTS
A total of 80 patients were randomized to receive either bilastine (n = 40) or fexofenadine (n = 40). Six patients lost to follow-up (one in bilastine group and five in fexofenadine group) and thus excluded from the final analysis. The baseline characteristics of patients are shown in [Table 1].
| Parameter | Bilastine (n=39) | Fexofenadine (n=35) | P value |
|---|---|---|---|
| Sex | |||
| Male | 22 | 18 | |
| Female | 17 | 17 | |
| Age (Mean±SD) years. | 36.43±11.81 | 37.34±10.83 | 0.7 |
| Disease duration (Mean±SD) months | 10.0±5.51 | 10.59±6.5 | 0.82 |
| Previous medications | |||
| First-generation antihistamines | 16 | 9 | |
| Second-generation antihistamines | 21 | 21 | |
| Corticosteroids | 2 | 4 | |
| Cyclosporine | 0 | 1 | |
| Urticaria severity | |||
| Mild | 12 | 11 | 0.95 |
| Moderate | 27 | 24 | |
| Initial UAS7 score (Mean±SD) | 17.66±3.97 | 18.34±3.94 | 0.47 |
| Baseline CU-Q2oL score (Mean±SD) | 49.23±8.53 | 51.2±9.40 | 0.35 |
Efficacy assessment
Control of urticaria
After 2 weeks of treatment, 58.97% (n = 23) of patients in bilastine group while 51.42% (n = 18) of patients in fexofenadine were having adequate control of urticaria. The difference in response rate was not statistically significant in both the groups (P = 0.51) [Figure 2].
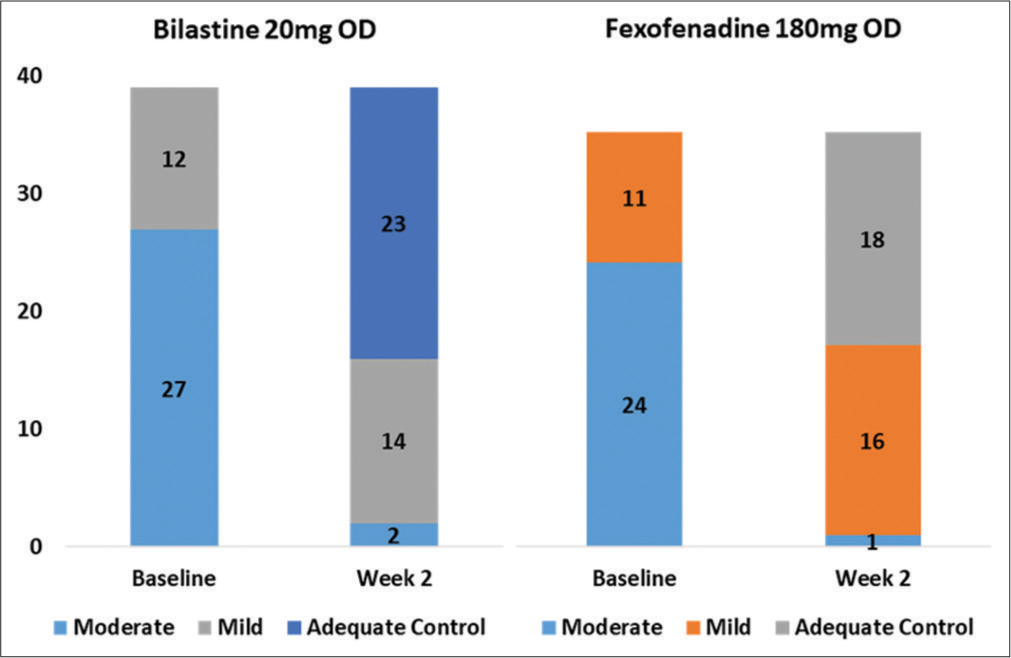
- Number of patients who were symptom free at week 2 in both the treatment groups.
Sixteen and 17 patients were non-responder to standard dose of bilastine and fexofenadine, respectively. These patients were updosed to bilastine (20 mg BD) and fexofenadine (180 mg BD) for the next 2 weeks, respectively. At the end of 4 weeks, 9/16 patients in the bilastine group and 8/17 patients in the fexofenadine group achieved adequate control of urticaria [Figure 3]. The difference in improvement was not statistically significant (P = 0.59).
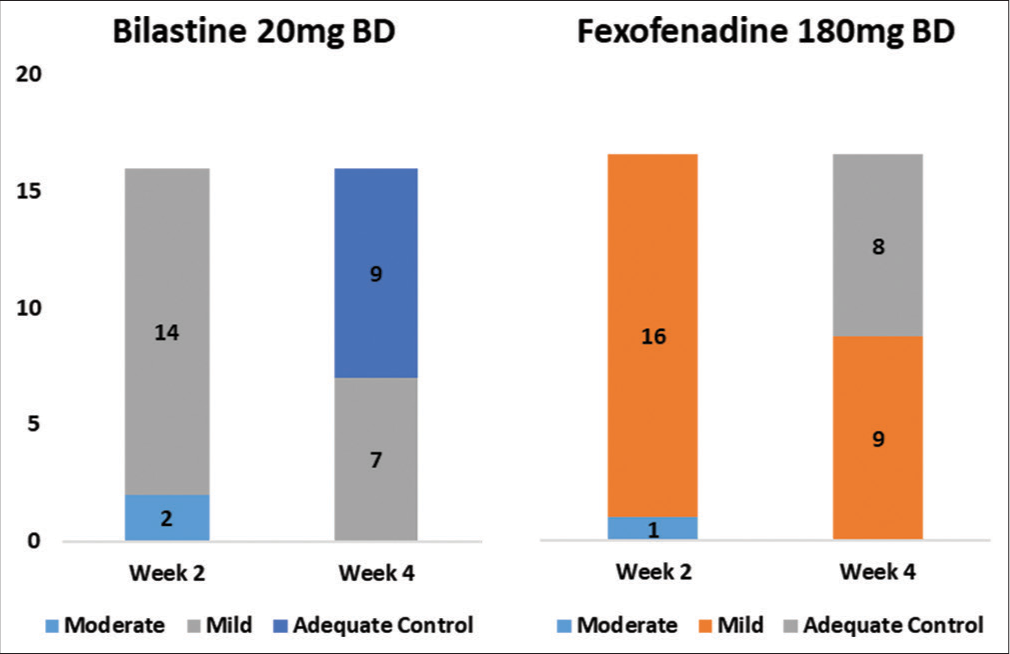
- Number of patients who achieved adequate control of urticaria at week 4 after updosing of bilastine and fexofenadine.
Improvement in mean UAS7 score
At baseline, the mean UAS7 score in the bilastine and fexofenadine group was 17.66 ± 3.97 and 18.34 ± 3.94, respectively. Following treatment with either bilastine or fexofenadine for 2 weeks, the mean UAS7 score fell to 7.85 ± 4.50 and 7.80 ± 3.76 in the bilastine group and fexofenadine group, respectively. Similar reduction in mean UAS score was observed at week 4 in both the groups (5.31 ± 3.01 vs. 5.65 ± 2.57). This improvement in UAS7 score from baseline in both the groups was statistically significant (P < 0.0001); however, the difference between the treatment groups was not significant (P = 0.96) [Figure 4].
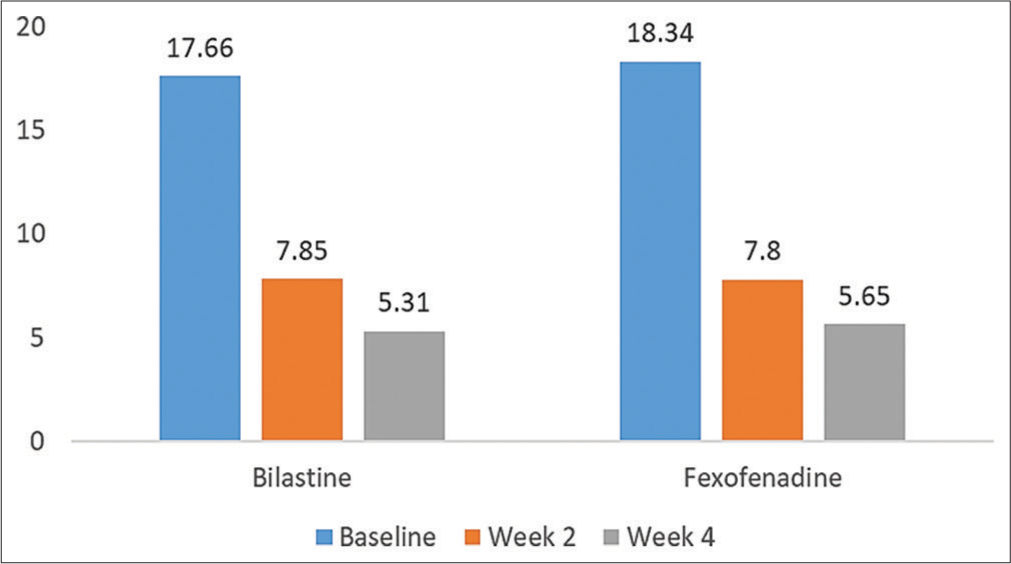
- Improvement in UAS7 score in both the treatments at week 2 and week 4.
Quality of life
Bilastine was associated with a significant improvement quality of life of patients compared to fexofenadine. At baseline, the mean CU-Q2oL score in the bilastine and fexofenadine group was 49.23 ± 8.53 and 51.2 ± 9.40, respectively. At week 2, bilastine was associated with a significant reduction in mean CU-Q2oL score compared to fexofenadine (37.31 ± 6.87 vs. 42.03 ± 7.05; P < 0.005). Similar statistically significant reduction in CU-Q2oL was observed in the bilastine group compared to the fexofenadine group at week 4 (32.38 ± 5.83 vs. 38.71 ± 5.92; P < 0.005) [Figure 5 and Table 2].
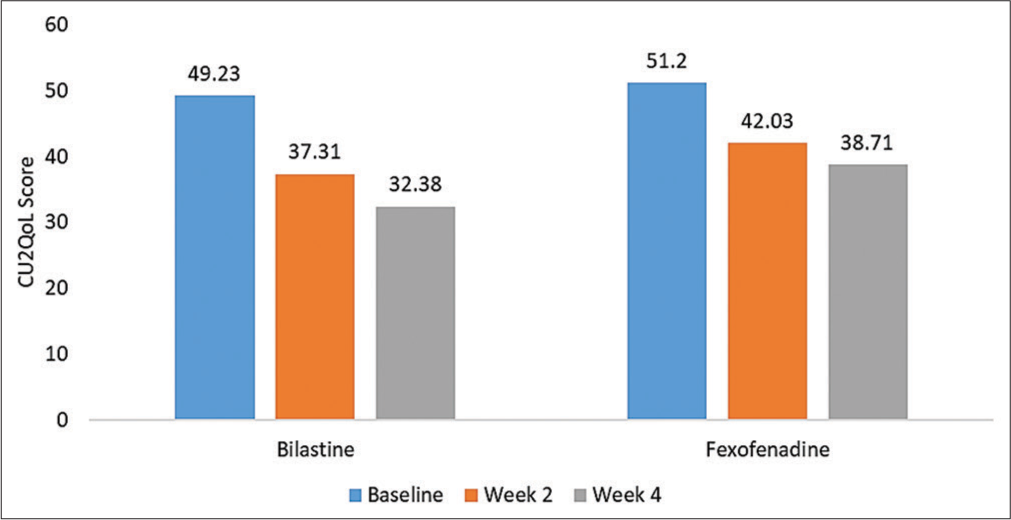
- Improvement in quality of life of patients assessed by CUQ2oL.
| Bilastine | Fexofenadine | P value | |
|---|---|---|---|
| Baseline | 49.23±8.53 | 51.2±9.40 | 0.35 |
| Week 2 | 37.31±6.87 | 42.03±7.05 | 0.005 |
| Week 4 | 32.38±5.83 | 38.71±5.92 | 0.005 |
Discomfort caused by urticaria
Urticaria-associated discomfort during the preceding week was measured using a VAS. In terms of VAS, there was a statistical difference between bilastine and fexofenadine at both visits [Table 3]. It suggests that bilastine is well accepted as a non-sedating antihistamine as compared to other by patients.
| Bilastine | Fexofenadine | P value | |
|---|---|---|---|
| Week 2 | 5.87±1.24 | 4.86±1.25 | 0.05 |
| Week 4 | 6.5±0.7 | 5.47±1.5 | 0.02 |
Somnolence
A major concern with increasing doses of H1-antihistamines is that of somnolence. Sleepiness with the drug was measured by VAS. On day 14, fexofenadine had a higher sedation score than bilastine. The somnolence score of bilastine and fexofenadine did not increase when their dose was increased [Figure 6]. Bilastine was statistically better (P < 0.05) than the fexofenadine as a non-sedating antihistamine.
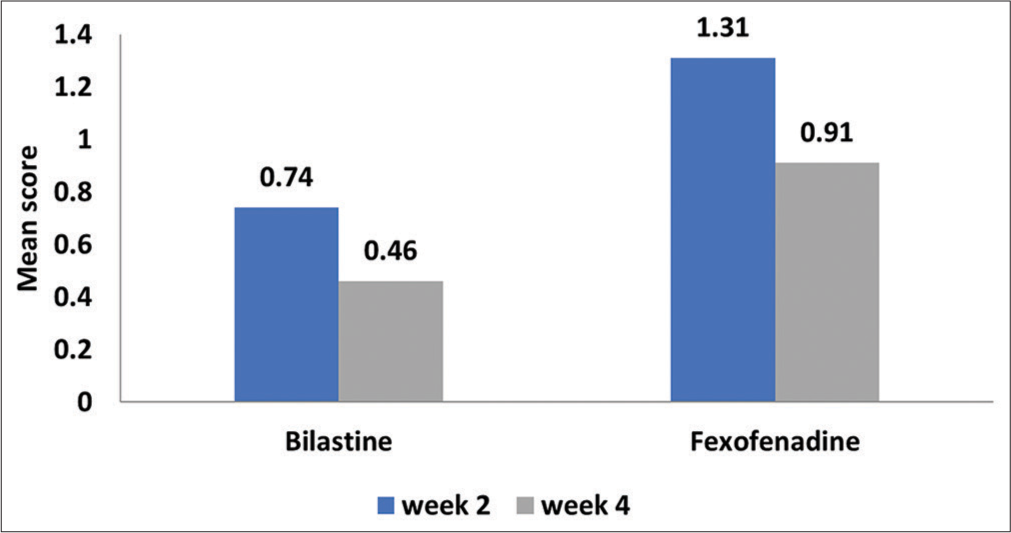
- Sleepiness with the drugs during the study period.
Safety assessment
Overall, both the treatments were well tolerated. A total of 13 patients (six in the bilastine group and seven in the fexofenadine group) reported one or more AEs during the study period. Sedation was the most common AE followed by nausea, headache, and fatigue. All the AEs were of mild-to-moderate severity. None of the patient discontinued the treatment due to AEs [Figure 7].
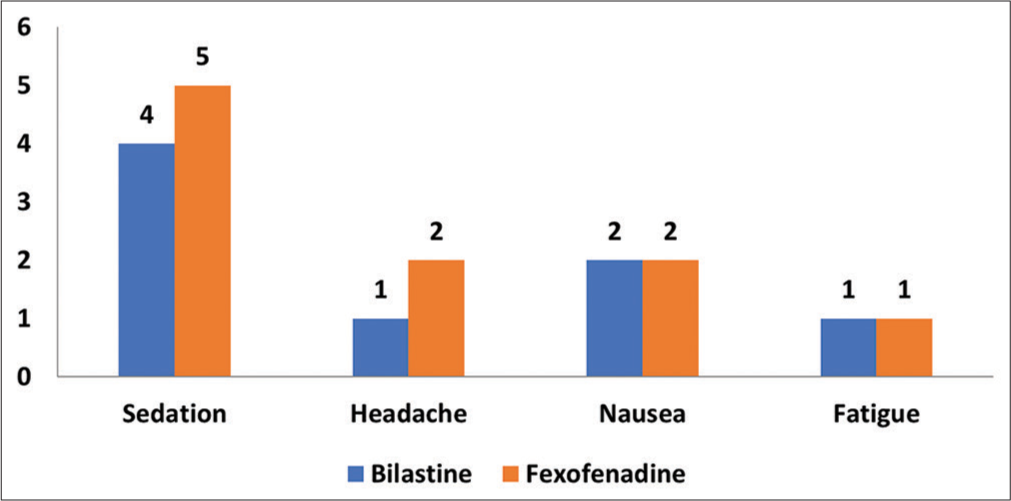
- Adverse events reported during the study period in both the groups.
DISCUSSION
SGAHs are the first-choice drugs in the management of CU. However, more than 50% of patients do not respond adequately to licensed dosage of SGAHs.[5-10] In such patients’, various guidelines recommend updosing of SGAHs.[1-4]
However, studies directly comparing effectiveness and safety of SGAHs are limited, especially in Indian setup.
This study compares the effectiveness and tolerability of bilastine and fexofenadine at a standard dose for 14 days followed by updosing of bilastine and fexofenadine in patients not responding to standard dosage either drugs. This study provides evidence that in patients with difficult to treat CU, increasing the daily dose of two SGAHs, bilastine and fexofenadine, to up to 2 times their conventionally prescribed doses increase the control of urticaria symptoms without compromising patient safety.
In our study, there was a significant improvement in UAS7 score in both the treatment groups with 58.97% and 51.42% of patients achieving adequate control of urticaria in the bilastine and fexofenadine group, respectively, at standard dosage. These results are in accordance with the previous published studies for individual drugs.[13-16,19-22]
Although all the patients included in the study were using some or other form of previous therapies with poor control of urticaria, changing the therapy to either bilastine or fexofenadine was associated with improvement in control of urticaria. This suggests that both bilastine and fexofenadine are highly efficacious in the management of urticaria as suggested in the previous studies.
About 56.25% of patients in the bilastine group and 47.05% in the fexofenadine group achieved adequate control of urticaria after doubling the dose of respective drug. This clearly suggests that, in patients with difficult to treat urticaria, updosing of SGAHs is associated with increase in control of urticaria. These results are in accordance with the previous studies.[17,18,23-25]
Weller et al. in their retrospective study reported that updosing of bilastine was an effective approach in majority of patients with CSU.[17] Similarly, Krause et al. concluded that updosing of bilastine was associated with increased effectiveness.[18]
Magen et al. in their study reported a significant benefit while updosing to 2–3 tablets of fexofenadine in patients with CSU.[23] Godse et al. in their study concluded that fexofenadine in higher doses controlled urticaria in the majority of patients.[24] An uncontrolled clinical trial by Tanizaki et al. supported that increasing doses of fexofenadine from 120 mg to 240 mg daily reduced symptoms of CSU.[25] CU has a significant impact on HRQoL, thus improvement in quality of life of patient is very important goal of urticaria treatment. In our study, bilastine was associated with a significant improvement in quality-of-life patients as assessed by CU-Q2oL compared to fexofenadine. There are no comparative studies to assess the improvement in quality of life for bilastine and fexofenadine. However, both the drugs were associated with improvement in quality-of-life patients with urticaria in the previous studies.[13,16,26,27]
Faster and longer duration of action, lowest H1RO in the CNS compared to other SGAHs,[12] and additional anti-inflammatory activity[27] may be associated with the increased improvement in quality of life of patient with bilastine compared to fexofenadine.
Perhaps, the major outcome of this study was the finding that patients did not experience increased somnolence when stepping up their daily dose as opposed to an anticipated increase in somnolence on the basis of reports that all second-generation H1-antihistamines may cause a small degree of sedation.
It is well known that somnolence is one of the most reported unwanted effects of antihistamines. However, in our study, patients on bilastine did not experience increased somnolence when stepping up their daily dose. Moreover, patients in the bilastine arm had lower somnolence than fexofenadine on both visits, days 14 and 28. It could be due to tolerance to somnolence which can develop within 4 days of subsequent use of H1-antihistamines.
Both the treatments in our study were well tolerated, none of the patient discontinued the therapy due to AE. These results are in accordance with the previous studies.[13-16,19-22]
CONCLUSION
This study provides evidence that in patients with CSU, updosing of bilastine, a second-generation non-sedating antihistamine, is effective, well-tolerated, and less sedating than updosing of fexofenadine. Updosing (increasing the daily dose) of bilastine provided relief from urticaria symptoms, improved quality of life in the majority of the patients without compromising somnolence or safety. The availability of such clinical evidence assists the physicians in choosing an appropriate treatment for their patients with CSU.
Acknowledgments
The authors are thankful to Glenmark Pharmaceuticals for donating the trial medications free of cost to the researchers. The authors are also grateful to the faculty, residents, and staff of the department of dermatology for their cooperation during the entire duration of the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Gaurav Deshmukh, Dr. Dhiraj Dhoot, and Dr. Hanmant Barkate are employees of Glenmark Pharmaceuticals Ltd, Mumbai, India.
References
- The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226-36.
- [CrossRef] [PubMed] [Google Scholar]
- ATTENTUS, a German online survey of patients with chronic urticaria highlighting the burden of disease, unmet needs and real-life clinical practice. Br J Dermatol. 2016;174:892-4.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus statement for the diagnosis and treatment of urticaria: A 2017 update. Indian J Dermatol. 2018;63:2-15.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of chronic spontaneous urticaria with an inadequate response to H1-antihistamines: An expert opinion. Eur J Dermatol. 2017;27:10-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bilastine: A new H1-antihistamine with an optimal profile for updosing in urticaria. J Eur Acad Dermatol Venereol. 2017;31:1447-52.
- [CrossRef] [PubMed] [Google Scholar]
- Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: A systematic review and meta-analysis. Br J Dermatol. 2016;175:1153-65.
- [CrossRef] [PubMed] [Google Scholar]
- Management of chronic spontaneous urticaria in routine clinical practice following the EAACI/GA(2)LEN/EDF/WAO guidelines. Actas Dermosifiliogr. 2017;108:346-53.
- [CrossRef] [PubMed] [Google Scholar]
- The characteristics of urticaria in 390 patients. Br J Dermatol. 1998;138:635-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis of chronic spontaneous urticaria in 117 patients not controlled by a standard dose of antihistamine. Allergy. 2013;68:229-35.
- [CrossRef] [PubMed] [Google Scholar]
- H1-antihistamine up-dosing in chronic spontaneous urticaria: Patients' perspective of effectiveness and side effects a retrospective survey study. PLoS One. 2011;6:e23931.
- [CrossRef] [PubMed] [Google Scholar]
- Bilastine: A lifetime companion for the treatment of allergies. Curr Med Res Opin. 2020;36:445-54.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: A multi-centre, double-blind, randomized, placebo-controlled study. Allergy. 2010;65:516-28.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of bilastine in Japanese patients with chronic spontaneous urticaria: A multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II/III study. Allergol Int. 2017;66:317-25.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of bilastine on chronic spontaneous urticaria refractory to levocetrizine: Real world experience in India. Dermatol Ther. 2021;34:e14557.
- [CrossRef] [Google Scholar]
- Effectiveness, safety, and tolerability of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic spontaneous urticaria: A double-blind, parallel group, randomized controlled trial. Dermatol Ther. 2020;33:e13946.
- [CrossRef] [Google Scholar]
- Updosing of bilastine is effective in moderate to severe chronic spontaneous urticaria: A real-life study. Allergy. 2018;73:2073-5.
- [CrossRef] [PubMed] [Google Scholar]
- Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy. 2013;68:921-8.
- [CrossRef] [PubMed] [Google Scholar]
- A double-blind, placebo-controlled trial of fexofenadine HCl in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1999;104:1071-8.
- [CrossRef] [Google Scholar]
- Comparative efficacy of fexofenadine and levocetirizine in chronic idiopathic urticaria. Indian J Dermatol. 2007;52:212-3.
- [CrossRef] [Google Scholar]
- Once-daily fexofenadine treatment for chronic idiopathic urticaria: A multicenter, randomized, double-blind, placebo-controlled study. Ann Allergy Asthma Immunol. 2005;94:662-9.
- [CrossRef] [Google Scholar]
- Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2000;84:517-22.
- [CrossRef] [Google Scholar]
- Antihistamines do not inhibit the wheal induced by the intradermal injection of autologous serum in resistant chronic idiopathic urticaria. Allergy Asthma Proc. 2012;33:531-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fexofenadine in higher doses in chronic spontaneous urticaria. Indian Dermatol Online J. 2010;1:45-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the efficacy of fexofenadine 120 mg and 240 mg per day on chronic idiopathic urticaria and histamine-induced skin responses in Japanese populations. J Dermatolg Treat. 2013;24:477-80.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of fexofenadine hydrochloride on productivity and quality of life in patients with chronic idiopathic urticaria. Cutis. 2007;79:157-62.
- [Google Scholar]
- In vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonist. Drugs R D. 2006;7:219-31.
- [CrossRef] [PubMed] [Google Scholar]






