Translate this page into:
Efficacy and safety of crisaborole ointment in pediatric atopic dermatitis: A 4-week open-label study
*Corresponding author: Abhishek De, Department of Dermatology, Calcutta National Medical College, Kolkata, West Bengal, India. dr_abhishek_de@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: De A, Chakraborty D, Grisilda B, Chaudhuri S, Godse K, Dhar S. Efficacy and safety of crisaborole ointment in pediatric atopic dermatitis: A 4-week open-label study. Indian J Skin Allergy. 2024;3:60-5. doi: 10.25259/IJSA_45_2023
Abstract
Objectives:
Managing mild-to-moderate atopic dermatitis (AD) often necessitates topical therapies, and one such recently introduced option is crisaborole ointment. This study sets out to assess the efficacy and safety of crisaborole ointment in pediatric cases of AD over four weeks.
Material and Methods:
Nineteen children between 2 and 16 years old with mild-to-moderate AD were enrolled and treated with crisaborole ointment twice daily in affected areas for 30 days. The primary objective was to appraise the shift in the investigator’s static global assessment (ISGA) scores (0–4) every week for the four-week follow-up. The severity of pruritus score (SPS) was another secondary objective. Furthermore, individual indicators of clinical signs that included erythema, exudation, excoriation, induration/papulation, and lichenification, were examined with subjective scores (0–3). Children’s dermatology quality of life index (CDLQI) was employed to study the quality of life.
Results:
Following four weeks of crisaborole ointment treatment, the average ISGA score declined from 2.58 ± 0.61 to 0.95 ± 0.78, signifying a substantial reduction in AD severity (P < 0.001). The SPS score also decreased from a mean of 2.32 ± 0.478 to 0.84 ± 0.60 (P < 0.001), underscoring a significant reduction in itching. Moreover, individual markers for clinical signs of AD, including erythema, exudation, excoriation, induration/papulation, and lichenification, all exhibited statistically significant improvement. Crisaborole ointment was well tolerated. Only 6 of the 19 patients reported a localized burning sensation, which was manageable. No patient needed to be withdrawn during the study period. The CDLQI showed a substantial drop in scores, decreasing from an average of 13.79 ± 3.57 at the commencement to 6.74 ± 1.97 (P < 0.001). Furthermore, 14 out of 19 patients met the study’s primary goal, achieving at least a 2-point reduction in ISGA along with the attainment of clear or nearly clear skin (ISGA 0–1).
Conclusion:
Our study found crisaborole ointment significantly improved pediatric AD symptoms and was well-tolerated. The only adverse event was localized burning in a few patients. Further, research is needed for validation.
Keywords
Crisaborole
Phosphodiesterase 4 inhibitor
Atopic dermatitis
Eczema
Children
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory disease presenting as intensely pruritic eczematous lesions. Most affected patients have mild-to-moderate disease severity. It affects both adults as well as children. AD-associated pruritus causes frequent scratching and significantly hampers the patients’ and their families’ quality of life.[1]
More than 80% of children experience the symptoms into adulthood. In addition, severe comorbidities, including asthma and allergic rhinitis, are frequently linked to AD.[2]
Even though there have not been any new molecules approved for the treatment of AD in the past 15 years, treatment guidelines still call for the use of topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), or both. Topical treatments are frequently prescribed to reduce inflammation and prevent flares. Despite its effectiveness, both these options have limitations in their utilization. To prevent striae formation, local cutaneous atrophy, and systemic adverse effects, prolonged TCS usage is prohibited, especially on sensitive areas with thin skin, such as the face and groin. TCIs can cause a burning sensation during application. Hence, it needs increased patient education.[3]
Phosphodiesterase 4 (PDE4) regulates inflammatory cytokine production in AD by degrading cyclic adenosine monophosphate (cAMP). Inflammatory cells in AD patients have higher PDE4 activity, and inhibiting PDE4 in monocytes in vitro reduces proinflammatory cytokines.[4]
While minimizing unintended side effects, a topical PDE4 inhibitor formulation may meet the demand for focused suppression of inflammation in the skin of AD patients. Topical crisaborole 2% is the first PDE4 inhibitor approved on December 14, 2016, for patients with mild to moderate AD over two years of age. Later, on March 24, 2020, it got extended approval from the United States Food and Drug Administration to use for three months and older children.[5] Crisaborole had been recently approved in India. This is the first approved topical prescription treatment for eczema in over a decade. To the best of our literature searches, there is no published data from the Indian subcontinent on the efficacy and safety of crisaborole in AD. Moreover, beyond the clinical trial data, there is very limited real-life evidence on the molecule in the management of AD, though it has found its place in the most recent AD treatment guidelines. Therefore, we conducted this open-label prospective study to assess the efficacy and safety of crisaborole ointment in pediatric cases of AD in Indian scenarios over four weeks.
MATERIAL AND METHODS
This was an open-label and prospective study conducted at a tertiary care hospital in Kolkata after approval of the Institutional Ethics Committee. The inclusion criteria for the study were patients of age two years or older, who had a clinical diagnosis of AD according to the Hanifin and Rajka criteria and who had 5% or more treatable body surface area involvement and a baseline investigator’s static global assessment (ISGA) score of mild (2) or moderate (3) [Table 1].
| Scale | Grade | Definition |
|---|---|---|
| 0 | Clear | No rash or marks, minor residual pigmentary changes; no erythema or induration and/or papulation; and no oozing/crusting |
| 1 | Almost clear | Mild pink erythema, minimal or no induration, and/or papulation and no oozing/crusting |
| 2 | Mild | Mild pink erythema with mild induration and/or papulation and no oozing/crusting |
| 3 | Moderate | Pink-red erythema with moderate induration and/or papulation with or without oozing/crusting |
| 4 | Severe | Dark red erythema with severe induration and/or papulation and with oozing/crusting |
The use of systemic corticosteroids or biologic therapy in the previous 28 days, as well as the use of TCS or TCI in the previous 14 days, was a major exclusion criterion. Patients who had active skin infections were not included in the study. Patients on stable regimens (constant use 14 days before day 1) of oral antihistamines and inhaled corticosteroids for the treatment of non-AD medical conditions were permitted to continue taking those drugs. In addition, patients were permitted to treat dry skin areas close to but not covering the curable AD-affected areas using appropriate bland emollients.
Patients were enrolled over two months and instructed to apply a thin layer of crisaborole ointment to cover each lesion twice daily to all areas affected by AD throughout the 29-day follow-up. The scalp area was excluded from treatment to avoid patient dissatisfaction with the application of ointment to the hair. Patients were scheduled for weekly visits to our outpatient departments on days 8, 15, 22, and 29.
The primary efficacy end point of success in ISGA score at day 29 was defined as clear (0) or almost clear (1) with a 2-grade or more improvement from baseline. ISGA was assessed at screening and on days 8, 15, 22, and 29. ISGA was assessed on a 5-point scale from clear (0) to severe (4).
The weekly change in the severity of pruritus score (SPS) was our secondary objective. The severity of pruritus was assessed on a 4-point scale from none (0) to severe (3) at screening and on days 8, 15, 22, and 29 [Table 2].
| Scale | Grade | Definition |
|---|---|---|
| 0 | None | No itching |
| 1 | Mild | Occasional, mild itching, and/or scratching |
| 2 | Moderate | Constant or intermittent itching and/or scratching that is not disturbing sleep |
| 3 | Severe | Constant severe itching and/or scratching that is disturbing sleep |
Furthermore, individual indicators of clinical signs, which included erythema, exudation, excoriation, induration/papulation, and lichenification, were assessed on a 4-point subjective scale from none (0) to severe (3). These signs of AD were assessed at baseline and on day 29 [Table 3]. Children’s dermatology quality of life index (CDLQI) was employed to study the quality of life.
| Score | Grade | Definition |
|---|---|---|
| Erythema (redness) | ||
| 0 | None | No redness |
| 1 | Mild | Barely detectable pink erythema |
| 2 | Moderate | Dull red; clearly distinguishable erythema |
| 3 | Severe | Deep, dark red; marked and extensive erythema |
| Exudation (oozing and crusting) | ||
| 0 | None | No oozing or crusting |
| 1 | Mild | Minor signs of oozing |
| 2 | Moderate | Definite signs oozing or crusting |
| 3 | Severe | Marked and extensive oozing or crusting |
| Excoriation (evidence of scratching) | ||
| 0 | None | No evidence of excoriation |
| 1 | Mild | Mild excoriation |
| 2 | Moderate | Definite excoriation |
| 3 | Severe | Marked, deep, or extensive excoriation |
| Induration/papulation | ||
| 0 | None | None |
| 1 | Mild | Slightly perceptible elevation |
| 2 | Moderate | Clearly perceptible elevation but not extensive |
| 3 | Severe | Marked and extensive elevation |
| Lichenification (epidermal thickening) | ||
| 0 | None | No epidermal thickening |
| 1 | Mild | Minor epidermal thickening |
| 2 | Moderate | Moderate epidermal thickening; accentuated skin lines |
| 3 | Severe | Severe epidermal thickening; deeply accentuated skin lines |
The data were recorded in Microsoft 365 Excel and analyzed by statistical tests using GraphPad InStat.
A flowchart of the study design is shown in Figure 1.
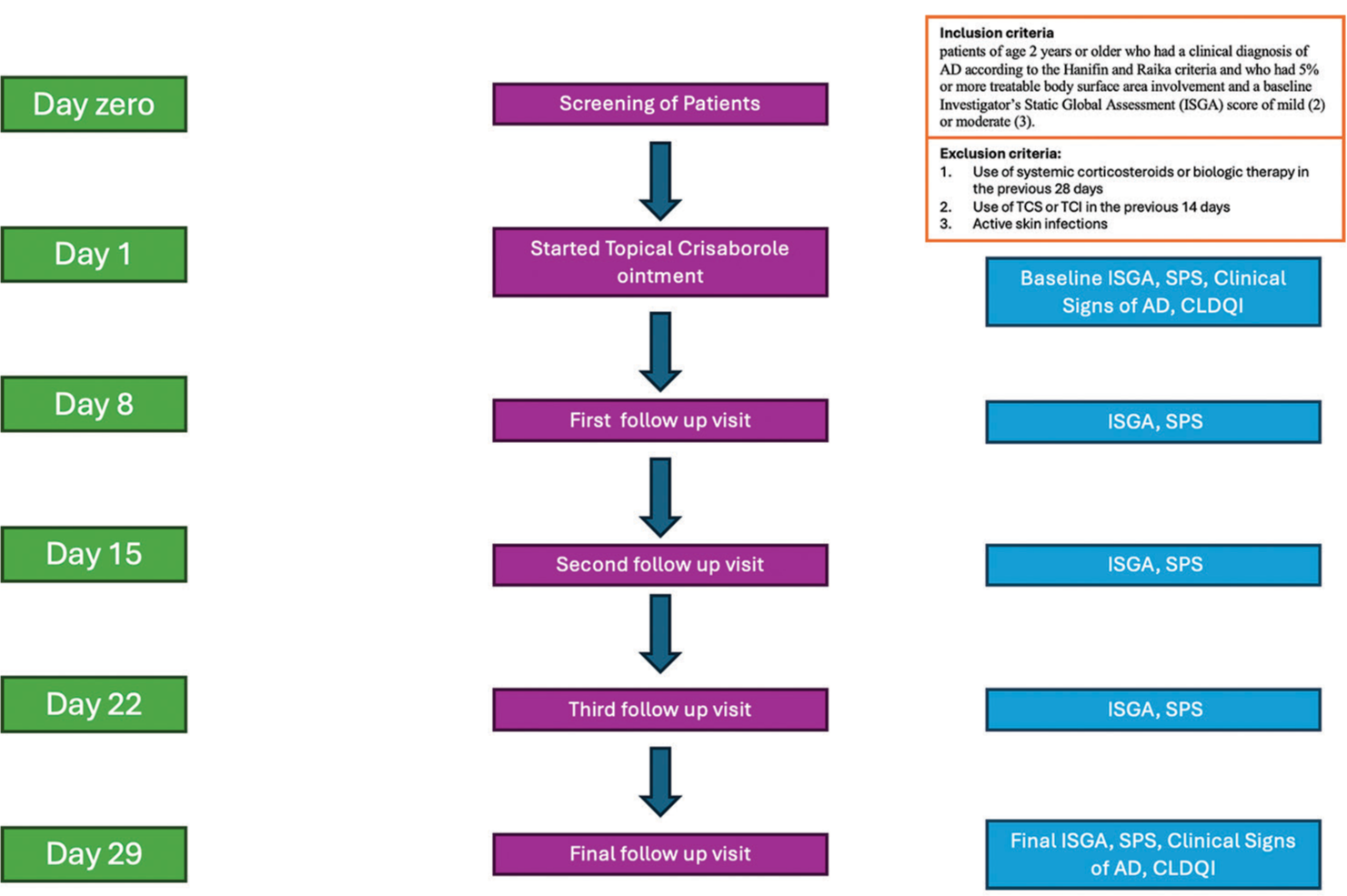
- Flowchart; TCS: topical corticosteroids; TCI: topical calcineurin inhibitors; AD: atopic dermatitis; CLDQI: Children’s dermatology quality of life index; SPS: severity of pruritus score.
RESULTS
A total of 19 patients fulfilling the inclusion criteria were included in the study. Out of the total 19 patients, 12 (63.2%) were male and 7 female (36.8%). The age range varied from 2 years to 16 years. Average age was 6.52 ± 4.22 years. Disease severity at baseline, measured by ISGA, was 2.58 ± 0.61, with the SPS at baseline being 2.32 ± 0.478. The markers of severity of AD at baseline were measured by already described scoring systems,and those were erythema (2.16 ± 0.3), exudation (1.53 ± 0.51), excoriation (1.84 ± 0.60), induration/papulation (1.84 ± 0.60), and lichenification (1.21 ± 0.79).
Following four weeks of crisaborole ointment treatment, the average ISGA score declined from 2.58 ± 0.61 to 0.95 ± 0.78, signifying a substantial reduction in AD severity [Figure 2]. The result of the paired-t test indicated that there is a significantly large difference between baseline (M = 2.58, standard deviation [SD] = 0.61) and post-treatment (M = 0.95, SD = 0.78), t (18) = 7, P < 0.001 (P = 0.000001467) severity scores; normality P-value was 0.02629 and priori power was 0.5409, post hoc power was 1, skewness was 0.2213, and the skewness shape was potentially symmetrical.
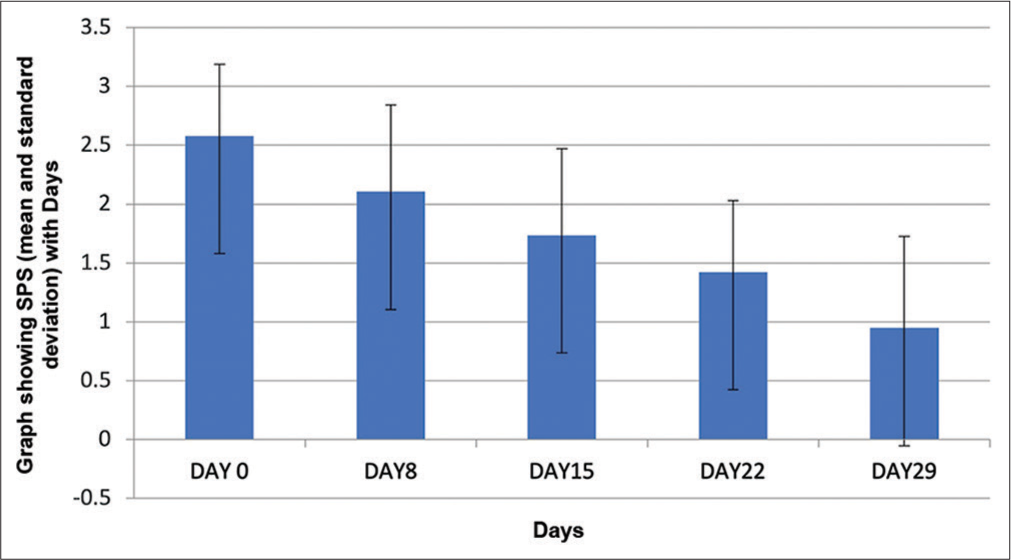
- The graph shows the change in mean investigator’s static global assessment (ISGA) score from baseline and at the end of 29 days which is statistically significant (P < 0.001).
The SPS score also decreased from a mean of 2.32 ± 0.478 to 0.84 ± 0.60, underscoring a significant reduction in itching [Figure 3]. The two-tailed paired t-test value was <0.0001.
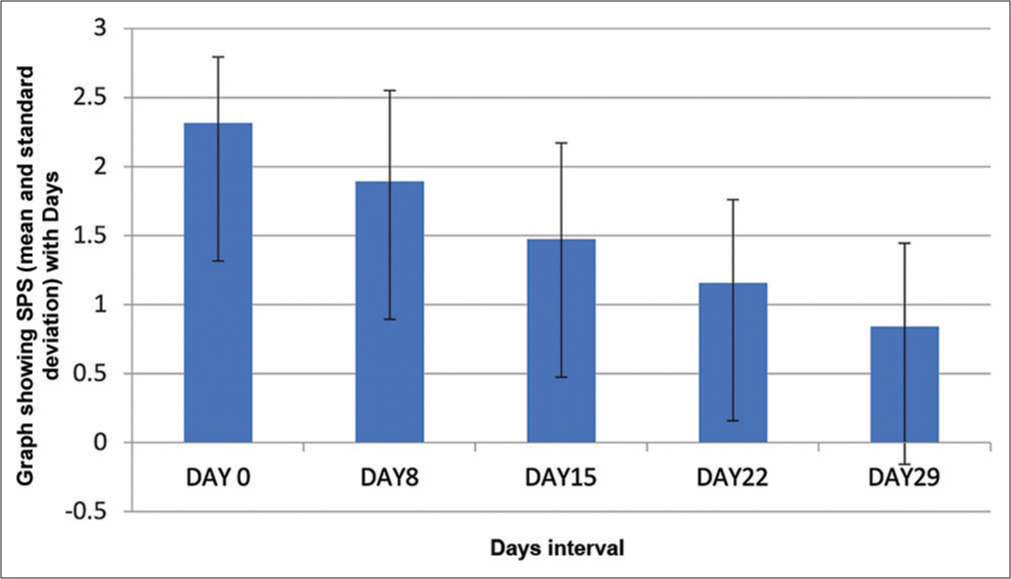
- It shows the change in mean severity of pruritus score (SPS) score from baseline and at the end of 29 days which is statistically significant (P < 0.0001).
Individual markers for clinical signs of AD, including erythema, exudation, excoriation, induration/papulation, and lichenification, all exhibited statistically significant improvement at the end of the study, that is, 29 days [Figure 4]. Erythema score reduced from 2.16 ± 0.37 at baseline to 1.05 ± 0.71 on day 29. Exudation score reduced significantly from 1.53 ± 0.51 at baseline to 0.37 ± 0.49 on day 29. Similarly, the excoriation score,which was 1.84 ± 0.60 at baseline, dropped to 0.79 ± 0.54 on day 29. Another marker of clinical signs of AD, induration/papulation, improved from a baseline level of 1.84 ± 0.60 to a day 29 level of 0.84 ± 0.60. Lichenification that is often regarded as a hallmark of chronic eczema, also improved from a baseline score of 1.21 ± 0.79 to 0.42 ± 0.51. The two-tailed paired t-test results of all these showed P < 0.0001.
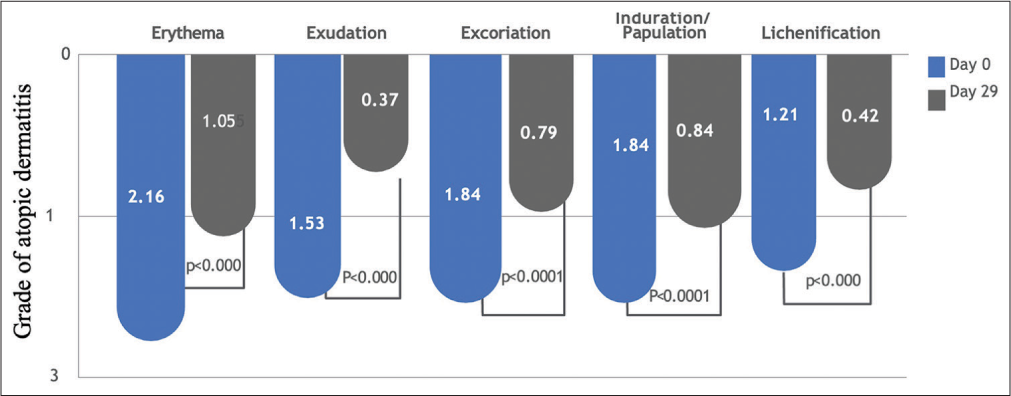
- The graph shows the change in mean scores of erythema excoriation exudation induration and lichenification from baseline and at the end of 29 days which is st (002).
Crisaborole ointment was well-tolerated in our subset of patients; only six of the 19 patients reported a localized burning sensation that improved in most patients with continuous use of the ointment. No patient needed to be withdrawn during the study period.
The CDLQI showed a substantial drop in scores, decreasing from an average of 13.79 ± 3.57 at the commencement to 6.74 ± 1.97 (P < 0.001; [Figure 5]). Furthermore, 14 out of 19 patients met the study’s primary goal, achieving at least a 2-point reduction in ISGA, along with the attainment of clear or nearly clear skin (ISGA 0–1) [Figures 6, 7a and b].
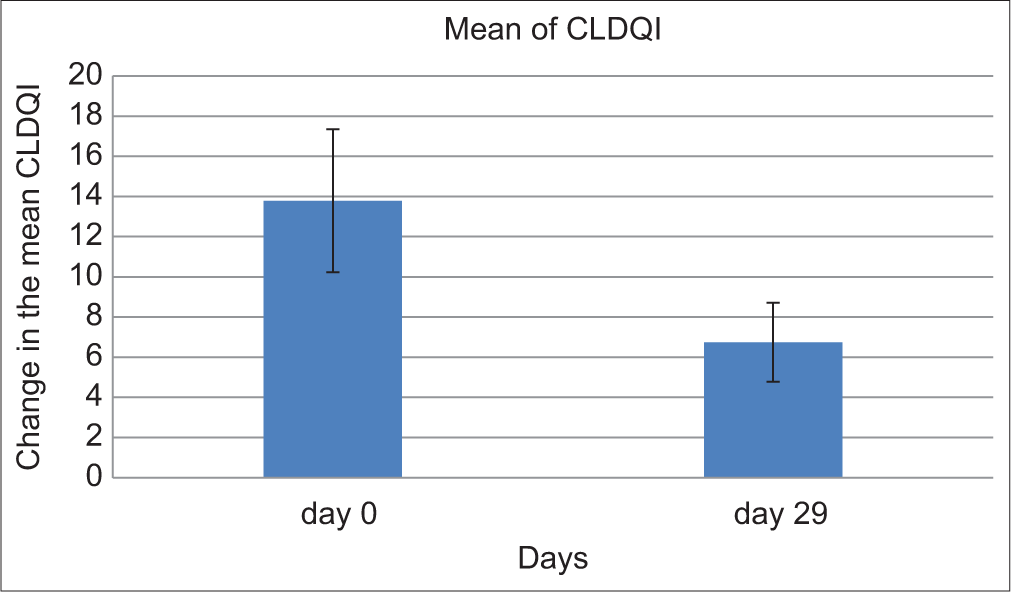
- It shows the change in mean children’s dermatology quality of life index (CDLQI) score from baseline and at the end of 29 days which is statistically significant (P < 0.0001).
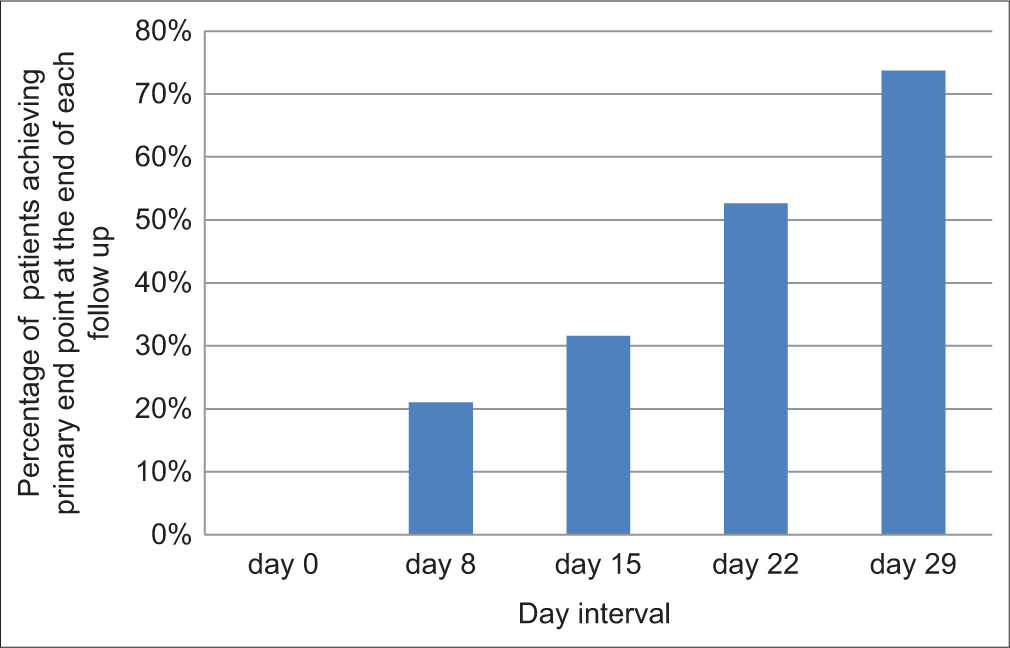
- The graph shows that the percentage of patients achieving primary end point increased at the end of each follow-up period, i.e., days 8, 15, 22, and 29.
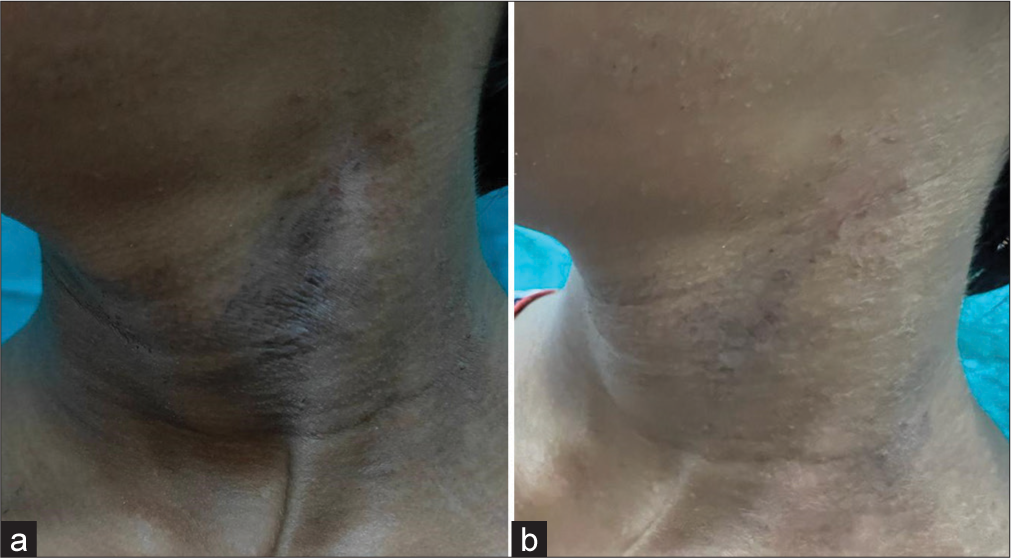
- (a and b) It shows the change in atopic dermatitis lesion before and after treatment with crisaborole ointment.
DISCUSSION
PDE4 activity is elevated in circulating inflammatory cells of AD skin. Its inhibition decreases the production of cytokines that cause inflammation. Crisaborole’s unique boron chemistry allows for the generation of a low-molecular-weight molecule that promotes efficient skin penetration. PDE-4 inhibition by crisaborole results in increased intracellular cAMP levels that improves cellular control of inflammation. This results in suppression of the release of proinflammatory cytokines by the downstream regulation of the nuclear factor-kB pathway.[6] Bissonnette et al.[7] conducted a unique intra-patient randomized controlled trial to delineate the mechanism of action of crisaborole. They evaluated the change in levels of AD biomarkers on lesional biopsy specimens on days 0, 8, and 15 and found significant downregulation of genes of Th2, Th17, and Th22 pathways. However, the exact mechanism of how a PDE4 inhibitor works in AD is incompletely understood.
Crisaborole is rapidly and substantially metabolized to inactive metabolites, limiting its systemic exposure and lowering the risk of negative effects.[8] Early clinical data showed a positive safety profile for crisaborole in children as young as two years of age, and preclinical studies in rats and mice established that crisaborole is non-carcinogenic.[5] To assess the effectiveness and safety of crisaborole 2% ointment in patients with mild-to-moderate AD, phase III studies were conducted in the Western and Japanese populations. Both the treatment groups that is the crisaborole and the vehicle-treated group, individually demonstrated significant improvement in terms of clinical parameters and attained the primary and secondary efficacy endpoints. Then, a subsequent comparison demonstrated greater improvement and a superior safety profile in the crisaborole group. Hence, it is noteworthy that the vehicle used plays a pivotal role in improvement. Although the vehicle does not contain the active ingredient, the filler or bulking agent used has favorable effects on AD lesions. This is mainly by improvement in the barrier function and counteracting the innate immune dysfunction. Many compounds, such as petrolatum, which is a commonly used vehicle, have an antimicrobial action, decrease the T-cell invasion, and also increase the epidermal thickness.[9] We conducted this study to evaluate the efficacy and safety of crisaborole on the Indian population, and it is the first of its kind.
The use of crisaborole ointment dramatically decreased AD signs and symptoms. Its positive efficacy profile was based on three factors: (1) early and persistent improvement in pruritus; (2) reduction in AD signs and symptoms starting within the first week of treatment; and (3) improvement in disease severity in the first two weeks of treatment. It reduces pruritus, which is crucial for treating AD since itching and scratching can worsen AD symptoms. Thus, it improves the quality of life scores.
It is necessary to find a safe and effective topical alternative to TCS and TCI due to their potential negative side effects and restricted long-term use. Crisaborole is a promising therapeutic substitute for currently available topical medicines due to its low systemic absorption and swift conversion to its inactive metabolites, minimizing the risk of systemic side effects.
Paller et al. conducted two identical vehicle-controlled (crisaborole: Vehicle, 2:1) double-blinded randomized controlled trials on patients aged two years or above. Both showed significant improvement in the crisaborole-treated group, in terms of ISGA scores along with minimum side effects.[5] Another phase II study on Chinese and Japanese populations aged two years and above used endpoints other than the ISGA score, such as Eczema Area and Severity Index total score and Peak Pruritus Numerical Rating Scale at week four. Crisaborole-treated population demonstrated significant improvement in terms of all criteria, along with a favorable safety profile.[10,11]
Since individuals with skin of color have unique clinical and genetic features of AD, treatment outcomes may vary. We conducted this study due to the dearth of specific Indian data. We used ISGA, SPS, and CDLQI scores along with individual parameters such as erythema, excoriation, exudation, lichenification, and induration. Even though this study is on a small population, the promising results show that crisaborole is a unique and effective non-steroidal treatment option for mild to moderately severe patients of AD of Indian origin. However, a randomized controlled trial of crisaborole is required on a larger group comparing it with other non-steroidal topical treatments such as topical tacrolimus or pimecrolimus to delineate its exact role in the current algorithm of the management of AD.
Limitation
Our study was an open-label and single-center pilot study to evaluate the safety and efficacy of the molecule in the management of AD in Indian patients. We believe that a multicentric randomized control trial with a larger sample size against other non-steroidal treatments, such as tacrolimus or pimecrolimus, will give better information regarding the safety, efficacy, and positioning of the molecule in the management algorithm of AD.
CONCLUSION
The study of crisaborole ointment can be an important option for the treatment of mild-to-moderate patients with AD. The unique mechanism of the molecule may open the use of the molecule for other immuno dermatological disorders. However, we believe that our study is the first Indian data for the effectiveness and safety of the molecule in mild-to-moderate AD and should be considered by treating physicians as an alternative to topical steroids and calcineurin inhibitors.
Ethical approval
The research/study is approved by the Institutional Ethics Committee at CNMC, Kolkata, number EC/CNMC/356, dated 23.09.2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Abhishek De, Dr. Kiran Godse, Dr. Sandipan Dhar are on the Editorial Board of the journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Current burden of atopic dermatitis in India: A systematic literature review. Indian J Dermatol. 2023;68:487.
- [Google Scholar]
- A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-90.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines on management of atopic dermatitis in India: An evidence-based review and an expert consensus. Indian J Dermatol. 2019;64:166-81.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast titration: Real-world Indian experience. Clin Dermatol Rev. 2021;5:183-6.
- [CrossRef] [Google Scholar]
- Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- [CrossRef] [PubMed] [Google Scholar]
- Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis, and other chronic inflammatory diseases. Dermatol Ther (Heidelb). 2013;3:1-15.
- [CrossRef] [PubMed] [Google Scholar]
- Crisaborole and atopic dermatitis skin biomarkers: An intrapatient randomized trial. J Allergy Clin Immunol. 2019;144:1274-89.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in the development of phosphodiesterase-4 inhibitors. Eur J Med Chem. 2023;250:115195.
- [CrossRef] [PubMed] [Google Scholar]
- Crisaborole topical ointment, 2%: A nonsteroidal, topical, anti-inflammatory phosphodiesterase 4 inhibitor in clinical development for the treatment of atopic dermatitis. J Drugs Dermatol. 2016;15:390-6.
- [Google Scholar]
- Magnitude of benefit for topical crisaborole in the treatment of atopic dermatitis in children and adults does not look promising: A critical appraisal. Br J Dermatol. 2018;178:659-62.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of crisaborole ointment in Chinese and Japanese patients aged =2 years with mild-to-moderate atopic dermatitis. J Dermatol. 2023;50:847-55.
- [CrossRef] [PubMed] [Google Scholar]







