Translate this page into:
Cutaneous adverse drug reactions in children with a focus on hypersensitivity to systemic drugs: A narrative review
*Corresponding author: Uwe Wollina, Department of Dermatology and Allergology, Städtisches Klinikum Dresden, Dresden, Germany. uwollina@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chiriac AE, Pinteala T, Chiriac A, Wollina U. Cutaneous adverse drug reactions in children with a focus on hypersensitivity to systemic drugs: A narrative review. Indian J Skin Allergy. 2024;3:2-11. doi: 10.25259/IJSA_14_2024
Abstract
Drug hypersensitivity in children is rare compared to adults. Children more frequently develop an infection-associated exanthema. Nevertheless, about 95% of children with drug hypersensitivity present with mucocutaneous involvement. Early recognition of typical clinical symptoms is important to reduce morbidity and mortality. We review relevant type I and type IV disorders of drug hypersensitivity in children and discuss their differential diagnoses and treatment.
Keywords
Child
Diagnosis
Differential
Drug Hypersensitivity
Exanthema
Hypersensitivity
Delayed
Morbidity
INTRODUCTION
Drug hypersensitivity in children is rare compared to adults. Children more frequently develop an infection-associated exanthema, which can be misinterpreted as an adverse drug reaction. The UK Medicines and Health-care products Regulatory Agency reported that 14.7% of adverse drug reactions in the years 2000–2009 were observed in children <17 years. The main source of information came from nurses.[1] Antibiotics are on the first rank of responsible drugs in children: 1–10% of children treated with beta-lactams experience a skin rash with a recurrence risk of <10%.[2,3] The common antibiotics involved are vancomycin, cloxacillin, amoxicillin, ampicillin, meropenem, ciprofloxacin, and cefixime. In a survey from Puducherry, India, urticaria and cutaneous rashes were the leading type of adverse drug reactions. Children <1 year were the most vulnerable population.[4] The prevalence of adverse drug reactions among children was 18.4% at a university hospital in Cape Town, South Africa.[5] In neonatal intensive care units, preventable adverse drug event rates are between 0.47 and 14.38/1000 patient-days with antibiotics commonly involved.[6] In a recent study from Serbia and Croatia, antibiotics were responsible for adverse drug reactions in 83%, non-steroidal anti-inflammatory drugs (NSAIDs) in 8.4%, and analgoantipyretics in 3.8%. Cutaneous involvement was observed in 96.2%.[7]
Drug-related hypersensitivity is classified as immediate or non-immediate (delayed) depending on the time of onset during treatment. Immediate reactions typically appear within 1–6 h after the last drug administration. In contrast, non-immediate commonly occurs after several days of treatment.[8]
The four severe adverse drug reactions, that is, drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens– Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and acute-generalized exanthematous pustulosis (AGEP), are not common among children and infants, but they are potentially life-threatening. In a multicenter trial from Turkey (n = 58), the final diagnosis was SJS/TEN in 60.4%, DRESS in 27.6%, followed by AGEP in 12%. Drugs were responsible in 93.1% of the patients, with antibiotics (51.7%) and antiepileptics (31%) as the most common triggers.[9]
Recent studies suggest that adverse drug reactions in children are generally underreported. Possible reasons for this might be that about 65% of prescribed drugs in pediatric patients are off-label and that dosage errors occur in about 31%.[10,11]
TYPE I ALLERGIC REACTION (IMMEDIATE, IMMUNOGLOBULIN E [IGE]-MEDIATED)
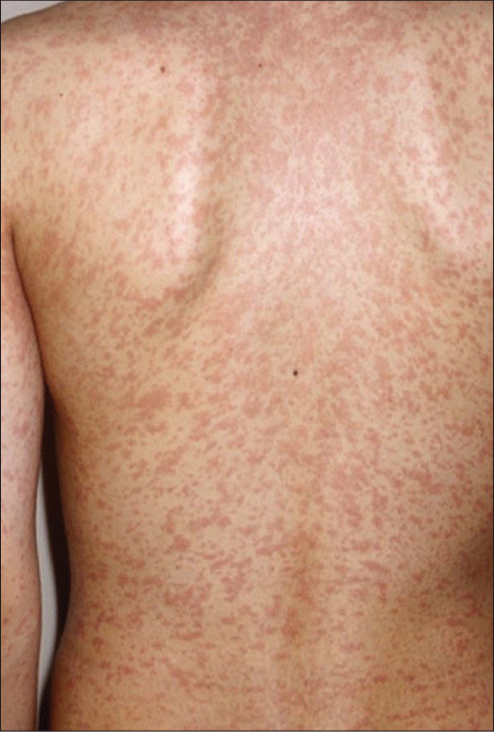
This type of allergic reaction is mediated by antigen-specific-IgE which binds to mast cells and causes mast cell degranulation. Mast cell degranulation syndrome manifests clinically quickly, immediately or in the first h after exposure (in the case of toxidermia secondary to drug intake). The specific clinical pictures are acute urticaria and/or angioedema. Sometimes, in children, urticarial lesions have an intense erythematous-ecchymotic appearance and are ring-shaped [Figure 1]. Drug skin reaction may be preceded by palmoplantar pruritus, with or without erythema, a suggestive sign of an allergic reaction. The other signs of mast cell degranulation may be present, in varying degrees of severity. Any drug can cause such a reaction, however, beta-lactams, paracetamol, NSAIDs, and antibiotics are the most common.[3]

- Urticarial drug-reaction type I.
Anaphylaxis is an acute systemic reaction involving symptoms of a type-I allergic reaction that can involve the whole body and is potentially fatal. In children, foods are much more frequently involved than drugs – in contrast to adults. A recent study suggested that IgE-mediated food-protein allergy affects 7.6% of children in the US.[12] Venoms account for about 1/5th of cases, while drugs are responsible for only 7%. Mast cells and basophils become activated. Systemic mastocytosis is a risk factor for severe anaphylaxis. Severity grades are summarized in Table 1.[13] It is characteristic that after a short prodromal stage, there is a sudden involvement of at least two organ systems.
| Grade | Skin and general subjective symptoms | Abdomen | Respiratory tract | Cardiovascular |
|---|---|---|---|---|
| I | Itch | - | - | - |
| Flush | ||||
| Urticaria | ||||
| Angioedema | ||||
| II | Itch | Nausea | Rhinorrhea | Tachycardia (increase ≥20/min) |
| Flush | Cramps | Hoarseness | ||
| Urticaria | Vomitus | Dyspnea | Hypotension (decrease ≤20mm Hg systolic pressure) | |
| Angioedema | Arrhythmia | |||
| III | Itch | Vomiting | Laryngeal edema | Shock |
| Flush | Defecation | Bronchospasm | ||
| Urticaria | Cyanosis | Angioedema | ||
| Angioedema | ||||
| IV | Itch | Vomiting | Respiratory arrest | Cardiac arrest |
| Flush | Defecation | |||
| Urticaria | ||||
| Angioedema |
The classification is made according to the most severe symptoms observed but no symptom is mandatory
ALLERGIC REACTION TYPE IV (DELAYED TYPE)
It is a cell-mediated reaction through T lymphocytes; it manifests itself a few hours up to several days after drug exposure. Depending on the clinical manifestations, the pathogenic mechanism, and the latency period, the following entities have been described: maculopapular exanthema, acute generalized exanthematous pustulosis (AGEP), drug hypersensitivity syndrome (DRESS), Stevens–Johnson/Lyell syndrome (toxic epidermal necrolysis), and fixed drug eruption.
Delayed drug-induced exanthema
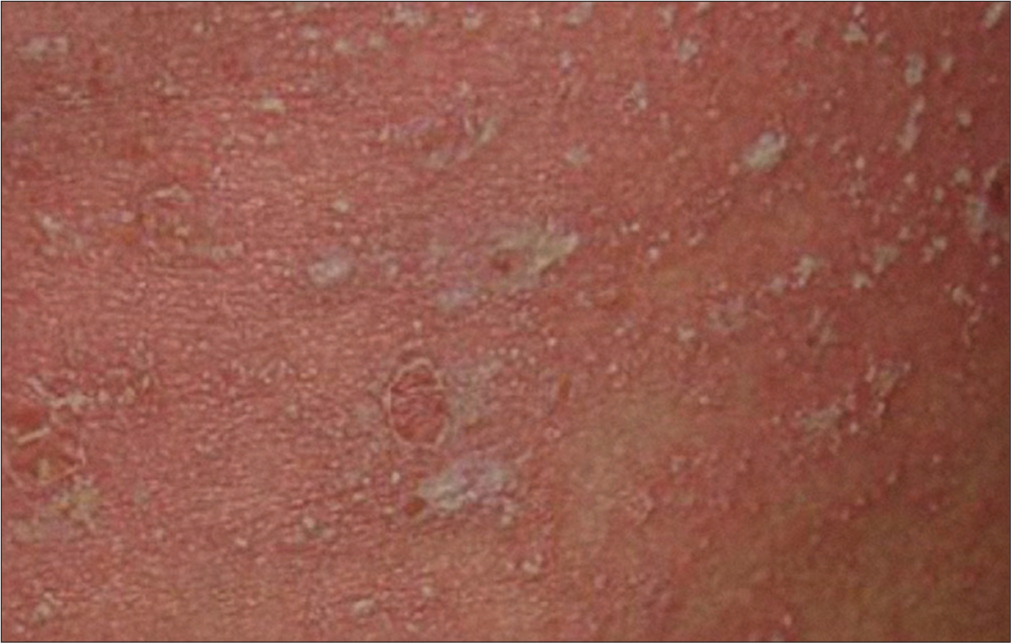
The rash appears 4–14 days after taking the drug (most often on the eighth day). The most often incriminated drugs in children are antiepileptics (carbamazepine and phenytoin) and antibiotics (penicillin, cephalosporins, and sulfonamides). The clinical picture can be either morbilliform, maculopapular, or erythema multiforme-like with a fixed character, interposed with areas of healthy skin, and located on the trunk and the root of the limbs, while face involvement is rare [Figure 2]. The cutaneous lesions are associated with pruritus. Elements in favor of the diagnosis are the absence of signs of severity, the absence of fever, the lack of changes in the mucous membranes and skin detachment, and favorable evolution in a few days after stopping the administration of the drug in question.[14]
- Delayed maculopapular drug eruption.
It is very difficult to differentiate drug-induced maculopapular exanthema and erythema multiforme-like reactions from the viral ones. Viral infections predominate in childhood and only 10%–20% of cases have been confirmed to be of drug origin. The most common viral infections responsible for maculopapular exanthema are caused by Measles virus, Rubella, Herpes simplex 6, Epstein Barr, Cytomegalovirus, and Enteroviruses. The risk of an exanthema also increases after the administration of a medical drug (e.g., amoxicillin administered in the case of an infection with the Epstein Barr virus).[14] Exanthemas occurring <72 h after starting a new drug medication are more likely to be due to viral infections. For differential diagnoses see Table 2.
| Viral rash | Drug rash | |
|---|---|---|
| Clinical findings | Pink to read macules/papules Starting from the trunk Symmetrical distribution Similar cases in family, kindergarten or school |
Pink to read macules/papules Drugs in medical history In case of 1stdrug contact delayed onset of rash but longer duration |
| Pathogenesis | Early onset but short duration of rash Cells are modified upon virus binding and uptake leading to a cytotoxic T cells response |
Cells are modified upon virus binding and uptake leading to a delayed T-cells immune response type IVb |
| Laboratory | Lymphopenia | Eosinophilia Allergy tests for confirmation after complete recovery |
Acute generalized exanthematous pustulosis (AGEP)
AGEP is very rarely reported in children, possibly also due to frequent confusion with a skin infection. The onset is acute, about 1–4 days after drug administration. The most frequently involved drugs reported are antibiotics, especially amoxicillin. It has been shown recently that mutations in interleukin (IL)-36RN, encoding the IL-36 receptor antagonist, and overexpression of IL-36g occur more frequently in AGEP patients. It represents a type IV d immune reaction.[15]
Clinical diagnostic criteria for AGEP are listed in Table 3. The score interpretation is as follows:
≤0 = Not AGEP;
1–4 = Possible AGEP;
5–7 = Probable AGEP;
8–12 = Definitive AGEP.[16]
| Characteristics | Score |
|---|---|
| Morphology | |
| Pustules | |
| Typical | +2 |
| Compatible with disease | +1 |
| Insufficient | +0 |
| Erythema | |
| Typical | +2 |
| Compatible with disease | +1 |
| Insufficient | +0 |
| Distribution/Pattern | |
| Typical | +2 |
| Compatible with disease | +1 |
| Insufficient | +0 |
| Post-pustular desquamation | |
| Yes | +1 |
| No/insufficient | +0 |
| Course | |
| Mucosal involvement | |
| Yes | −2 |
| No | +0 |
| Acute onset within 10 days of exposure | |
| Yes | +0 |
| No | −2 |
| Resolution within 15 days | |
| Yes | +0 |
| No | −4 |
| Fever (temperature ≥38°C) | |
| Yes | +1 |
| No | +0 |
| PMNs >7000/mm3 | |
| Yes | +1 |
| No | +0 |
| Histology | |
| Consistent with other disease | −10 |
| Not representative/no histology | +0 |
| Exocytosis of PMNs | +1 |
| Subcorneal and/or intraepidermal non-spongiform or NOS pustule (s) with papillary edema OR subcorneal and/or intraepidermal spongiform or NOS pustule (s) without papillary edema | +2 |
| Spongiform subcorneal and/or intraepidermal pustule (s) with papillary edema | +3 |
AGEP: Acute generalized exanthematous pustulosis, NOS: Not otherwise specified, PMNs: Polymorphonuclear leukocytes
The typical clinical picture is illustrated in Figure 3. Laboratory investigations show leukocytosis with neutrophilia. The histological examination of the skin biopsy (rarely recommended in current practice) can reveal the presence of intraepidermal (subcorneal) pustules, dermal edema, perivascular infiltrate with neutrophils and/or eosinophils, and foci of keratinocyte necrosis.[16]
- Acute generalized exanthematous pustulosis (AGEP) with multiple pustules, some coalescent, on an erythematous skin.
Skin tests are very important to confirm the diagnosis, since bacterial or viral infections can coexist (vicious circle: infection-drug-reaction type). Differential diagnoses are often difficult, especially with pustular psoriasis, scarlet fever, staphylococcal scaled skin syndrome (SSSS), and SJS or pustular DRESS.
The evolution resolves spontaneously within 1–2 weeks after discontinuing the administration of the drug. A fine, superficial, and “collaret”-type desquamation can sometimes be observed residually.
SJS and Lyell syndrome (toxic epidermal necrolysis; TEN)
SJS and TEN are diagnosed primarily clinically.[17,18] These are severe type IV immune reactions, with high mortality, especially at the age of 0–5 years, but with low incidence: 0.5–6.3/100,000 cases, with mortality of 0.3% in case of SJS and 4.2% in case of Lyell syndrome.[19,20] SCORTEN is a risk validation model for in-hospital mortality of these patients.[21] Sensitivity is 100% but specificity reaches only 24% among adult patients.[22]
Clinical manifestations appear with a delay of 4–28 days after the start of treatment. Drug intake is blamed in 60– 100% of cases, especially sulfonamides, antiepileptics, and NSAIDs. The involvement of viruses is difficult to appreciate because viral particles can replicate rapidly in toxidermia and, on the other hand, viral infections are very common in children.[23] Idiopathic Lyell syndrome has been reported with an incidence of 5–17% in children, although nosological classification confusions (including confusion with erythema multiforme) are common.[24,25] Mycoplasma pneumoniae infections have been identified in SJS/TEN as the leading infectious cause in up to 20%.[26]
The prodromal phase is nonspecific and often mistaken for a viral condition. It manifests by pharyngitis, dysphagia, cough, pain in the oral cavity, and burning sensations in the eyes. After 48–72 h, the clinical picture becomes complicated with:
Fever
Painful skin lesions: pseudo-cockaded, erythemato-violet, purpuric, and vesicular-bullous lesions, which initially affect the trunk and the roots of the limbs, then may expand and generalize in the following days
Nikolsky sign, that is, epidermal detachment on simple skin contact (“en linge mouillé” aspect)
Erosive-ulcerative lesions on the mucous membranes (at least two mucous membranes affected): Conjunctivitis, hemorrhagic-ulcerative cheilitis, nasal, genital, and anal ulcerations. An ophthalmologist should be consulted within 24 h after diagnosis [Figure 4].
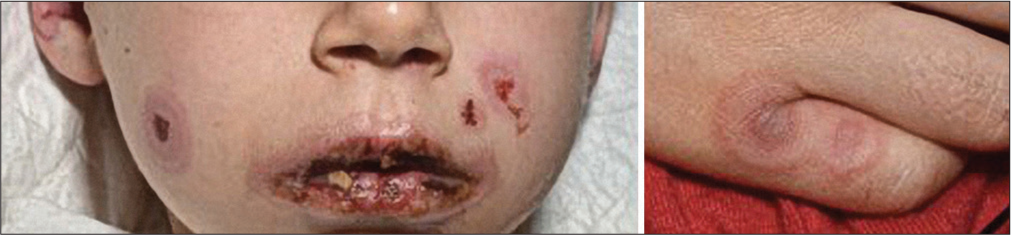
- Targetoid lesions in combination with ulcerating mucous lesions in SJS.
We can differentiate the two syndromes by the involved skin area:
SJS = Peeled or peelable area <10% skin surface
Lyell’s syndrome = Detached or detachable area >30% skin surface
Overlap syndrome = Detached or detachable area 10– 29%.[18]
Laboratory analyses are aimed at highlighting hydroelectrolytic disorders and visceral damage, especially pulmonary or digestive.
The detailed infectious assessment is mandatory:
Viral serology and blood polymerase chain reaction (PCR) for Epstein Barr virus, Cytomegalovirus, Herpes simplex virus 1 and 6, Parvovirus B19, Adenovirus, and Enterovirus
Serology: M. pneumoniae
• Nasopharyngeal exudate for respiratory viruses, M. pneumoniae, and Chlamydia pneumoniae
Skin PCR for herpes viruses, varicella-zoster virus, and Enterovirus.
Skin biopsy is mandatory and definitive for diagnosis. There is a massive epidermal keratinocyte necrosis, while direct immunofluorescence is negative excluding bullous autoimmune diseases.[19,20,23]
Identifying the culprit drug is very important, because discontinuing the suspected medication early helps to avoid further sequelae, especially ocular. In children, SJS/TEN can recur in about 18% since the precipitant in children is usually infection rather than drugs.[27]
The differential diagnoses is very important. The following disorders need consideration:
Polymorphous erythema: Skin lesions in a cocarde, with acral disposition and mucosal damage, caused by M. pneumoniae or herpes simplex virus
SSSS: Erythema predominates in the area of large folds and periorificial, areas of superficial detachment (Staphylococcus aureus toxins produce cleavage on desmoglein 1), and absence of mucosal lesions
Bullous autoimmune dermatoses: linear immunoglobulin A dermatosis and dermatitis herpetiformis, here the histological examination and immunofluorescence of a skin biopsy confirm the diagnosis
Adamantiades-Behҫet disease and Kawasaki syndrome
Burns
Phyto-photodermatitis: lesions located at the place of contact with the plant, in a warm and humid context.
For the latter, the subtype of photodistributed SJS/TEN is an important differential diagnosis.[28]
The differential diagnoses of SSSS and SJS/TEN is provided in Table 4.
| SJS/TEN | SSSS | |
|---|---|---|
| Clinical findings | Widespread blistering Nikolsky phenomenon positive Erythematous skin Base of blisters pink or white Less common in children |
Widespread blistering Nikolsky phenomenon positive Scarlatiniform erythema Base of blister with the same color as surrounding skin Most common in children <5 years |
| Histopathology | Subepidermal cleavage Necrotic keratinocytes (TEN) Scant inflammation |
Subcorneal cleavage Scant inflammation |
| Mortality rates | 0–16% | 2.6–11% |
| Pathogenesis | Mostly drug-related, CD8+cytotoxic lymphocyte delayed hypersensitivity, granulysin release | Exfoliative toxin from Staphylococcus aureusphage group II digests epidermal desmoglein 1 |
SJS: Stevens-Johnson syndrome, TEN: Toxic epidermal necrolysis, SSSS: Staphylococcal epidermolysis
Drug hypersensitivity syndrome (DRESS)
It is a severe form of adverse drug reaction, possibly underdiagnosed in children. Drugs that can induce DRESS are antiepileptics, antibiotics (sulfonamides, vancomycin), sulfasalazine, and NSAIDs. The period between the introduction of the medication and the onset of symptoms varies between 9 and 28 days, with a shorter period (about 9 days) in the case of antibiotics.
The clinical picture is specific, the child presents in the emergency unit with fever, asthenia, and a morbilliform rash that can rapidly progress to erythroderma. Facial edema and oral cavity lesions are characteristic and raise an alarm signal: Hemorrhagic-necrotic cheilitis, pharyngitis, and stomatitis [Figures 5 and 6]. The most frequently involved extracutaneous organ in children is the liver (affected in >80% of cases).[29]
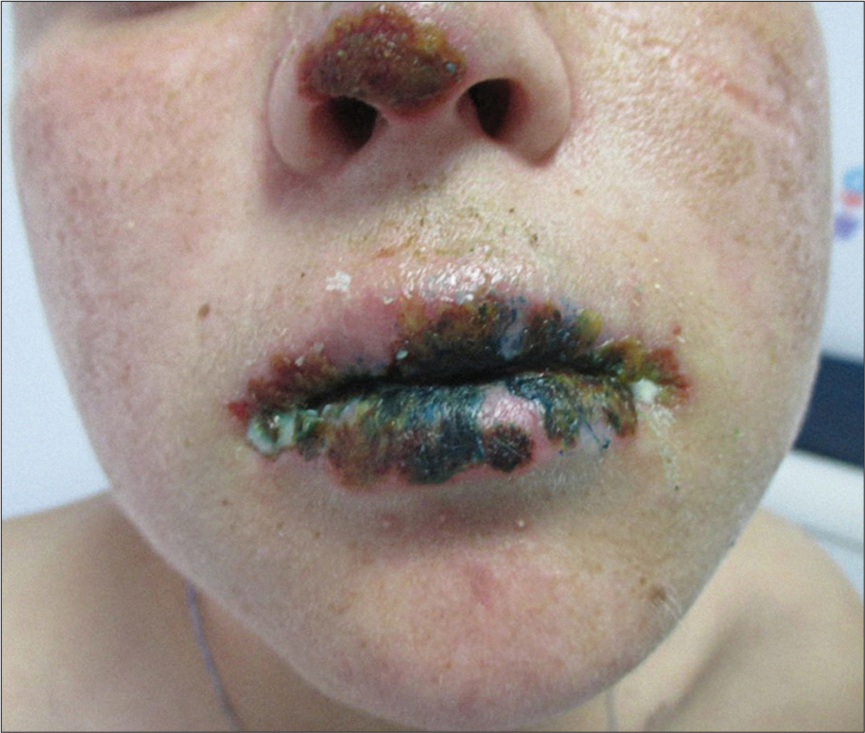
- Severe crusted lesions on the lips and on the nose tip in DRESS.
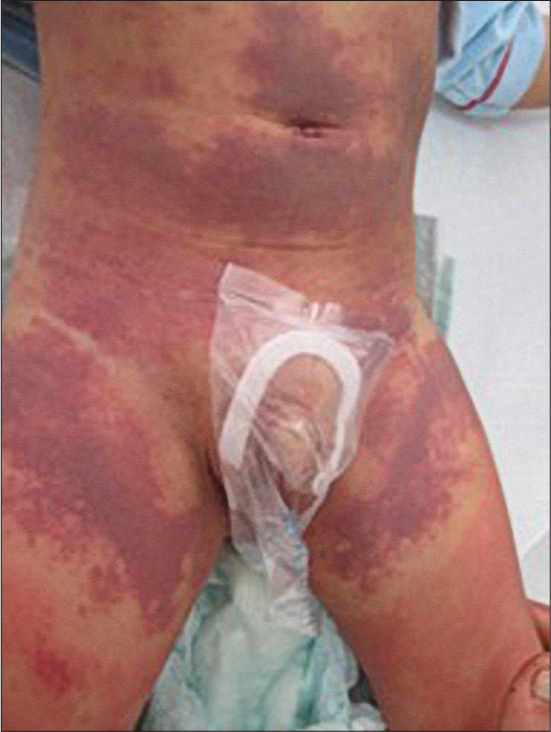
- Generalized erythema in DRESS.
Laboratory investigations should analyze peripheral blood for eosinophilia (>0.5 Gpt/L), liver and kidney function, and PCR in case of viral reactivation (very frequent in DRESS)-for Herpes virus 6 and 7, Cytomegalovirus, and Epstein Barr virus. Eosinophilia peaks with a delay of about 10 days after cutaneous eruption. The severity of the syndrome is determined by the systemic, multi-organ damage that occurs in over 90% of cases: cytolytic hepatitis, interstitial nephropathy, interstitial pneumopathy, myocarditis, and neurological damage. The risk of death is estimated at 10%, the risk of sequelae is also important, as is the risk of relapses.[30] Most often the disease recovery time is about two weeks. The severity can be evaluated using Registry of Severe Cutaneous Adverse Reactions DRESS score,[31] [Table 5].
| Parameter considered by Japanese consensus group criteria | The possible manifestations (for each parameter) that can satisfy a diagnosis of DiHS | †Point on RegiSCAR DRESS validation scoring system for features of atypical/typical DiHS | Minimum and maximum points that can be obtained by a patient with atypical/typical DiHS on RegiSCAR DRESS validation scoring system | |
|---|---|---|---|---|
| Minimum | Maximum | |||
| Latent period between onset of drug intake and appearance of symptoms | More than 3 weeks | 0 | 0 | 0 |
| Duration of clinical symptoms after withdrawal of the offending drug | Prolonged clinical symptoms after withdrawal of the offending drug | 0 | 0 | 0 |
| Fever | Fever | 0 | 0 | 0 |
| Maculopapular rash | 1. Maculopapular rash involving>50% of body surface area and does not satisfy features of rash suggestive of DRESS | +1 point for generalized maculopapular rash and−1 point for rash not showing 2/4 features suggestive of DRESS. Net score 0 | 0 | +2 |
| 2. Maculopapular rash and two of the four features among facial edema, rash resolving with psoriasiform desquamation, infiltrated skin lesions, and purpuric lesions on areas other than legs. | +1 point for generalized maculopapular rash and+1 point for rash showing 2/4 features suggestive of DRESS. Net score 2 | |||
| Internal organ involvement | 1. One internal organ involvement | +1 | +1 | +2 |
| 2. Two or more internal organ involvement | +2 | |||
| Hematological criteria | 1. Leukocytosis >11,000 cells/mm3 | 0 | 0 | +3 |
| 2. >5% atypical lymphocytes in peripheral smear | +1 | |||
| 3. Absolute eosinophil count >1500 cells/mm3 | +2 | |||
| 4. 1+2 | +1 | |||
| 5. 1+3 | +2 | |||
| 6. 2+3 | +3 | |||
| 7. 1+2 + 3 | +3 | |||
| *Cervical/generalized lymphadenopathy | 1. Cervical lymphadenopathy | 0 | 0 | +1 |
| 2. Generalized lymphadenopathy | +1 | |||
| *Human herpesvirus-6 reactivation | Human herpesvirus-6 reactivation | 0 | 0 | 0 |
| Total score | Atypical DiHS | 1 | 7 | |
| Typical DiHS | 1 | 8 | ||
RegiSCAR: Registry of severe cutaneous adverse reactions, DRESS: Drug reaction with eosinophilia and systemic symptoms, DiHS: Drug-induced hypersensitivity syndrome. *Features are not considered for diagnosing atypical DiHS. †If at least three out of the four tests were performed and found negative (antinuclear antibody, infection with hepatitis A, B, and C viruses, infection due to Mycoplasma/Chlamydia, and blood culture), one more point will be added on RegiSCAR scoring system
Fixed drug eruption
It is characterized by the appearance of an erythematous plaque, sometimes bullous, with well-defined, round edges that appear 24–48 h after the start of drug treatment, and rarely the lesions can be multiple [Figure 7]. Characteristic is the re-appearance in the same place on re-exposure to the culprit drug. Cases of generalized bullous fixed erythema have been rarely reported. The dominant drugs involved NSAIDs, paracetamol, and antibiotics such as trimethoprimsulfamethoxazole, penicillin, and tetracyclines.[32]
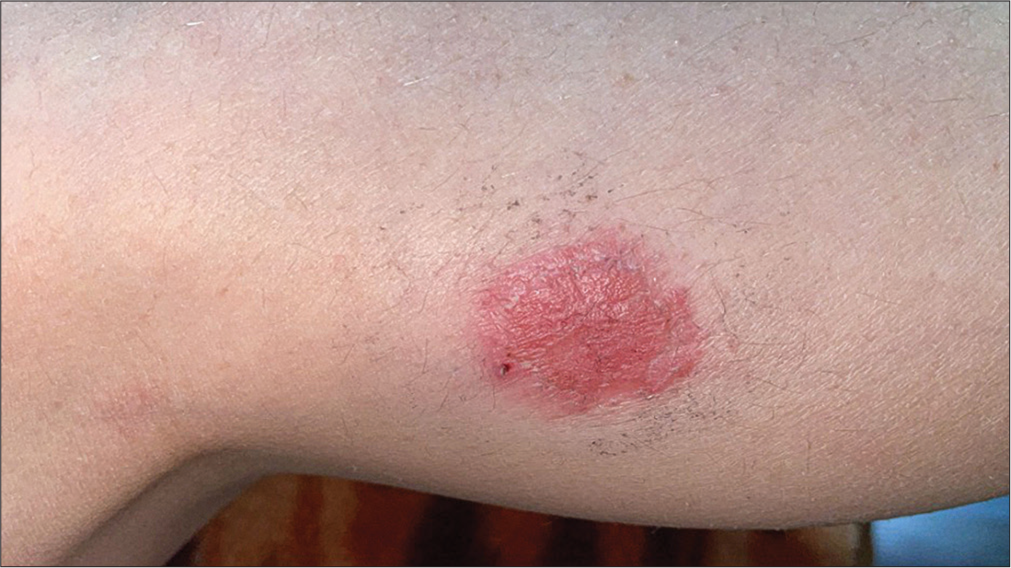
- Fixed drug eruption.
ALLERGY TESTING
The fundamental clue to the diagnosis of a drug hypersensitivity reaction is a careful and detailed medical history. Prophetic allergy testing is not recommended. The ALDEN score, an algorithm of drug causality for toxic epidermal necrolysis, can be used to improve the individual assessment of drug causality.[33]
According to European Academy of Allergy and Clinical Immunology recommendations, allergy testing must be carried out between six weeks and six months after successful treatment of drug reaction![34]
Suspect immediate allergy, acute urticaria or angioedema and/or anaphylaxis if the onset of symptoms is <1 h after drug administration. If the risk of drug allergy is high and requires allergy tests than specific IgE, prick test, and intradermal drug test can be performed. If the tests are negative and the post-drug reaction was not severe, an oral challenge test with the suspect molecule is proposed, but only under medical supervision.
In case of type-IV allergies, a patch test is recommended as a first-line skin test. It has a high specificity of 92% but a low sensitivity of 32%.[35] The frequency of positive patch test reactions depends on the culprit drug(s) and the type of cutaneous adverse reactions. The highest percentage of positive reactions has been seen in maculopapular exanthema.[36] Even in severe toxidermia and toxic epidermal necrolysis patch tests can be performed and considered to be safe.[37] The advantage of patch tests is that they can be done in an outpatient clinic and that commercialized forms of medical drugs can be used. In contrast to this, intradermal test can only be done with injectable drugs. Commercial skin test reagents are available only for a small number of drugs (i.e., penicillin and amoxicillin). The risk of severe allergic reactions by the test is higher and may warrant a stay in hospital.[38]
In maculopapular exanthema, erythema multiforme-like and urticarial reactions skin tests are positive in 10%. Patch test should be done first.[34,39]
Serum specific IgE assays against allergens are the most used in vitro diagnostic approach. They must be interpreted on the knowledge of the non-specific total IgE assay. Specific IgE is characteristic of type-I drug allergies. Other in vitro immunological laboratory methods (e.g., basophil activation test, basophil histamine release test, leukotriene release test (CAST), lymphocyte transformation test, lymphocyte activation test, and ELISpot test) are only available in specialized laboratories. A reliable confirmation or exclusion of drug hypersensitivity by in vitro tests only is impossible.[40] Nevertheless, the in vitro tests may be helpful when available and the interpretation of results is done with a sense of proportion. In addition, none of these in vitro diagnostic tools have a 100% negative predictive value.[41,42] Tables 4 and 5 summarize diagnostic approaches. Drug provocation test is a third-line diagnostic procedure. Risks and benefits should be discussed with the caregivers. The decision should consider the type of drug, severity of drug hypersensitivity reaction, if alternatives to the culprit drug are available and the necessity of drug therapy. The basis of provocation tests is applying the suspected drug substances in the form in which they caused the hypersensitivity reaction. Oral application is preferable compared with intramuscular or intravenous application.[43]
In case of suspected allergy to NSAIDs, NSAIDs-induced or -exacerbated food allergy needs to be excluded. Lipid-transfer protein Pru p 3 (found in peaches) is the most important known food allergen in such patients.[44] A summary of skin tests and in vitro tests in relation to the various cutaneous drug hypersensitivities is provided in Tables 6 and 7.[12]
| Drug reaction | Histology$ | Patch test | Prick test | Intradermal test |
|---|---|---|---|---|
| Acute urticaria* | - | - | (+) | (+) |
| Anaphylaxis** | - | (+) | + | + |
| Delayed exanthema | (+) | (+) | + | + |
| AGEP§ | + | + | - | - |
| SJS/TEN | + | + | + | + |
| DRESS | (+) | + | + | + |
| Fixed drug eruption§§ | + | - | + | + |
| Drug reaction | Specific IgE | BAT$ | CAST | LTT | ELISpot |
|---|---|---|---|---|---|
| Acute urticaria | + | (+) | (+) | (+) | - |
| Anaphylaxis* | + | + | + | + | - |
| Delayed exanthema | + | - | + | + | + |
| AGEP§ | - | - | + | + | + |
| SJS/TEN | - | (+) | + | + | + |
| DRESS | (+) | (+) | + | + | + |
| Fixed drug eruption§§ | - | - | - | - | - |
BAT: Basophil activation test, CAST: Cellular antigen stimulation test, LTT: Lymphocyte transformation test, ELISpot: Enzyme-linked immune absorbent spot assay. *In most cases of acute urticaria no skin tests are recommended, however in drug-induced cases this can be useful to confirm diagnosis. §In AGEP in vitro tests are often unnecessary. §§The most important diagnostic procedure is a scratch test in the lesion. AGEP: Acute generalized exanthematous pustulosis, DRESS: Drug reaction with eosinophilia and systemic symptoms, SJS: Stevens-Johnson syndrome, TEN: Toxic epidermal necrolysis.
TREATMENT
It is very important to recognize a drug skin reaction because they can be potentially life-threatening. Treatment is adapted to each clinical form. In fixed drug eruption and maculopapular exanthema emollients, antihistamines, and topical steroids are recommended.
Intramuscular application of adrenaline at a dose of 0.15– 0.6 mg outside of the upper thigh is the pharmacological treatment of first choice in anaphylaxis. Oxygen at a flow of 100% is recommended. Volume replacement to treat relative hypovolemia due to vasodilation and capillary leakage is necessary. Vital signs need careful monitoring. Patients with grade II or higher anaphylaxis should be hospitalized.[13]
Patients suspicious of toxic epidermic necrolysis should be hospitalized in a burn center. Treatment is multidisciplinary. The basic attempts aim at fluid replacement, nutrition, analgesia, skin care, eye care, urogenital care, and immunomodulatory treatment. The patients need monitoring for cutaneous, ocular, and systemic infections.[45]
Controversial results have been reported after administration of systemic steroids, cyclosporine, or intravenous immunoglobulins (IVIG).[21] In a systematic review and meta-analysis systemic corticosteroids were the only drug with a survival benefit for these patients.[46] In pediatric patients, a 10-year study of intensive unit treatment of TEN (n = 44) suggested that neither IVIG nor corticosteroids provided any mortality benefit compared to conservative treatment. The mortality rate in this study was 15.9%.[47]
The usual treatment for DRESS is systemic corticosteroid therapy with a slow tapering over a period of months, due to the risk of relapse. However, there has been evidence for cyclosporine A and JAK inhibitor tofacitinib in certain cases of DRESS.[48] In a study on four pediatric patients with severe DRESS, IVIG (total dosage: 1–2 g/kg) was effective in resolving fever within a median time of 1 (range, 0–5) day and cutaneous rash (clearance after 6.3 ± 1.6 days). Elevated liver enzymes also normalized and there was no mortality.[49] Further studies are needed.
AGEP sometimes requires local corticosteroid therapy, but the course is self-limited. Type-I acute urticaria and type-IV drug-induced exanthema can be treated by oral antihistamines and topical corticosteroids. In more severe cases, systemic corticosteroids may be necessary.[14]
CONCLUSION
Cutaneous adverse drug reactions are an important differential diagnoses of viral exanthema in children. Antibiotics, NSAIDs, antipyretics, and anticonvulsants are the systemic drugs more frequently involved in cutaneous adverse reactions. Early diagnosis is important to prevent sequelae from benign exanthem to severe drug reactions with significant morbidity and mortality. Clinical examination and detailed medical history are the fundamentals of diagnosis. Confirmation of diagnosis by skin tests and in vitro tests is recommended. Drug exposure is a third-line diagnostic tool reserved for hospital setting. There is an urgent need for validation and standardization of in vitro allergy tests in children. Treatment is based upon antihistamines and mostly topical corticosteroids, except for SJS/TEN and DRESS where systemic treatment is necessary.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Reported paediatric adverse drug reactions in the UK 2000-2009. Br J Clin Pharmacol. 2012;73:437-6.
- [CrossRef] [PubMed] [Google Scholar]
- The role of penicillin in benign skin rashes in childhood: A prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-22.
- [CrossRef] [PubMed] [Google Scholar]
- Short protocol for the study of paediatric patients with suspected betalactam antibiotic hypersensitivity and low risk criteria. Allergol Immunopathol. 2011;39:337-41.
- [CrossRef] [PubMed] [Google Scholar]
- A study of adverse drug reactions in pediatric patients. J Pharmacol Pharmacother. 2011;2:277-80.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse drug reactions in paediatric in-patients in a South African tertiary hospital. J Trop Pediatr. 2019;65:389-96.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and nature of medication errors and preventable adverse drug events in paediatric and neonatal intensive care settings: A systematic review. Drug Saf. 2019;42:1423-36.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosing single and multiple drug hypersensitivity in children: A tertiary care center retrospective study. Children (Basel). 2022;9:1954.
- [CrossRef] [PubMed] [Google Scholar]
- Tools for etiologic diagnosis of drug-induced allergic conditions. Int J Mol Sci. 2023;24:12577.
- [CrossRef] [PubMed] [Google Scholar]
- Severe cutaneous adverse drug reactions in pediatric patients: A multicenter study. J Allergy Clin Immunol Pract. 2017;5:757-63.
- [CrossRef] [PubMed] [Google Scholar]
- Physicians' perspectives on adverse drug reactions in pediatric routine care: A survey. World J Pediatr. 2022;18:50-8.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse drug reactions in newborns, infants and toddlers: pediatric pharmacovigilance between present and future. Expert Opin Drug Saf. 2012;11:95-105.
- [CrossRef] [PubMed] [Google Scholar]
- Management of food allergies and food-related anaphylaxis. JAMA. 2024;331:510-2.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update: S2k-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Medical Association of German Allergologists (AeDA), the Society of Pediatric Allergology and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Society for Neonatology and Pediatric Intensive Care (GNPI), the German Society of Dermatology (DDG), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Respiratory Society (DGP), the patient organization German Allergy and Asthma Association (DAAB), the German Working Group of Anaphylaxis Training and Education (AGATE) Allergo J Int. 2021;30:1-25.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced exanthemata: A source of clinical and intellectual confusion. Eur J Dermatol. 2010;20:255-9.
- [CrossRef] [PubMed] [Google Scholar]
- Culprit drugs induce specific IL-36 overexpression in acute generalized exanthematous pustulosis. J Invest Dermatol. 2019;139:848-58.
- [CrossRef] [PubMed] [Google Scholar]
- Acute generalized exanthematous pustulosis (AGEP)-a clinical reaction pattern. J Cutan Pathol. 2001;28:113-9.
- [CrossRef] [PubMed] [Google Scholar]
- Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis-diagnosis and treatment. J Dtsch Dermatol Ges. 2020;18:547-53.
- [CrossRef] [Google Scholar]
- Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States. J Am Acad Dermatol. 2017;76:811-7.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-54.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of SCORTEN in predicting mortality in toxic epidermal necrolysis. BMC Med Inform Decis Mak. 2022;22:273.
- [CrossRef] [PubMed] [Google Scholar]
- A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- [CrossRef] [PubMed] [Google Scholar]
- Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in Children: A pooled analysis. Pediatrics. 2009;123:e297-304.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence and outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatrics. 2011;128:723-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and outcomes in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Dermatol. 2022;49:895-902.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of children with recurrent episodes of Stevens Johnson syndrome. J Pediatric Infect Dis Soc. 2017;6:e140-3.
- [CrossRef] [PubMed] [Google Scholar]
- Photodistributed Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and proposal for a new diagnostic classification. Eur J Med Res. 2023;28:188.
- [CrossRef] [PubMed] [Google Scholar]
- Drug reaction with eosinophilia and systemic symptoms (DRESS): Series of 49 French pediatric cases. J Allergy Clin Immunol Pract. 2022;10:267-74.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: A retrospective cohort study from Taiwan. J Am Acad Dermatol. 2013;68:459-65.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083-4.
- [CrossRef] [PubMed] [Google Scholar]
- An update of fixed drug eruptions in Singapore. J Eur Acad Dermatol Venereol. 2015;29:1539-44.
- [CrossRef] [PubMed] [Google Scholar]
- ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60-8.
- [CrossRef] [PubMed] [Google Scholar]
- Drug hypersensitivity in children: Report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy. 2016;71:149-61.
- [CrossRef] [PubMed] [Google Scholar]
- The use of patch tests in the diagnosis of delayed hypersensitivity drug eruptions. Int J Dermatol. 2016;55:1219-24.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of drug patch tests in children. Allergy Asthma Proc. 2021;42:167-74.
- [CrossRef] [PubMed] [Google Scholar]
- Drug patch testing in Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review. Ann Allergy Asthma Immunol. 2023;130:628-36.
- [CrossRef] [PubMed] [Google Scholar]
- Drug patch testing in systemic cutaneous drug allergy. Toxicology. 2005;209:209-16.
- [CrossRef] [PubMed] [Google Scholar]
- Allergy testing in serious cutaneous drug reactions-harmful or beneficial? Contact Dermatitis. 1997;37:282-5.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline for allergological diagnosis of drug hypersensitivity reactions: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) in cooperation with the German Dermatological Society (DDG), the Association of German Allergologists (ÄDA), the German Society for Pediatric Allergology (GPA), the German Contact Dermatitis Research Group (DKG), the German Society for Pneumology (DGP), the German Society of Otorhinolaryngology, Head and Neck Surgery, the Austrian Society of Allergology and Immunology (ÖGAI), the Austrian Society of Dermatology and Venereology (ÖGDV), the German Academy of Allergology and Environmental Medicine (DAAU), and the German documentation center for severe skin reactions (dZh) Allergol Select. 2023;7:122-39.
- [CrossRef] [PubMed] [Google Scholar]
- Tools to improve the diagnosis and management of T-cell mediated adverse drug reactions. Front Med (Lausanne). 2022;9:923991.
- [CrossRef] [PubMed] [Google Scholar]
- IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020;13:100080.
- [CrossRef] [PubMed] [Google Scholar]
- Drug reexposure in children with severe mucocutaneous reactions. Allergol Immunopathol (Madr). 2022;50:104-7.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and updated classification of acute hypersensitivity reactions to nonsteroidal anti-inflammatory drugs (NSAIDs): NSAID-exacerbated or-induced food allergy. J Allergy Clin Immunol Pract. 2023;11:1843-53.
- [CrossRef] [PubMed] [Google Scholar]
- British Association of Dermatologists' guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in children and young people, 2018. Br J Dermatol. 2019;181:37-54.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. JAMA Dermatol. 2017;153:514-22.
- [CrossRef] [PubMed] [Google Scholar]
- Intensive care needs and long-term outcome of pediatric toxic epidermal necrolysis-a 10-year experience. Int J Dermatol. 2021;60:44-52.
- [CrossRef] [PubMed] [Google Scholar]
- First-line therapy in drug reaction with eosinophilia and systemic symptoms (DRESS): Thinking beyond corticosteroids. Front Med (Lausanne). 2023;10:1138464.
- [CrossRef] [PubMed] [Google Scholar]
- Successful intravenous immunoglobulin treatment in pediatric severe DRESS syndrome. J Allergy Clin Immunol Pract. 2018;6:1238-42.
- [CrossRef] [PubMed] [Google Scholar]







