Translate this page into:
Investigations and treatment of photodermatoses
*Corresponding author: Smitha S. Prabhu, Department of Dermatology and Venereology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India. smitha.prabhu@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Prabhu SS. Investigations and treatment of photodermatoses. Indian J Skin Allergy. 2024;3:45-53. doi: 10.25259/IJSA_6_2024
Abstract
The photodermatoses comprise of a miscellaneous set of photosensitive disorders with abnormal cutaneous response to sunlight. The diagnosis is mostly clinical and is augmented by methods such as phototesting, photoprovocation testing, and photopatch testing, which are discussed below. Treatment includes photoprotection, prophylactic phototherapy, as well as topical and systemic immunosuppression to varying extents. Various newer modalities for photoprotection as well as treatment are being explored.
Keywords
Phototherapy
Ultraviolet rays
Immunosuppression therapy
Urticaria
Patch test
INTRODUCTION
The photodermatoses comprise of a miscellaneous set of disorders, wherein sunlight or ultraviolet (UV) light exposure leads to abnormal cutaneous responses, and thereby visible cutaneous lesions. A simple classification of photodermatoses is given in Table 1.[1]
| Type | Examples |
|---|---|
| Idiopathic photodermatoses | PMLE, Juvenile Spring Eruption, AP, SU, HV, CAD, and AR |
| Caused by endogenous/exogenous agents | EPP, PCT, pseudoporphyria, phototoxicity, and photoallergy |
| Photo exacerbated conditions | SLE, dermatomyositis, pellagra, and disseminated superficial actinic porokeratosis |
| Photosensitive genodermatoses | Xeroderma pigmentosum, Cockayne syndrome, Bloom syndrome, Hartnup disease, Rothmund Thompson syndrome, and Kindler syndrome |
PMLE: Polymorphous light eruption, AP: Actinic prurigo, SU: Solar urticaria, HV: Hydroa vacciniforme, CAD: Chronic actinic dermatitis, AR: Actinic reticuloid, EPP: Erythropoietic protoporphyria, PCT: Porphyria cutanea tarda, SLE: Systemic lupus erythematosus
Although the clinical diagnosis of photodermatosis is often straightforward, there are fine nuances in the definitive diagnosis between the subsets. Accurate determination of the wavelength that causes the reaction may help in treatment as well as in preventing further relapses.
This article intends to focus on common conditions encountered in India such as polymorphous light eruption (PMLE), chronic actinic dermatitis (CAD), and photocontact dermatitis.
APPROACH TO A PATIENT WITH SUSPECTED PHOTODERMATITIS
In an ideal circumstance, this consists of establishing the diagnosis of photodermatitis, the specific subset involved, as well as determining the action spectrum responsible and identifying any offending photosensitive agent.
A detailed history and clinical evaluation are essential for the former, with careful questioning as to the latent period between sun exposure and occurrence of the lesions, and any seasonal and daytime variation. For example, lesions caused despite protection by window glass suggest involvement of ultraviolet A (UVA). Certain conditions such as juvenile spring eruption occur only during the spring or summer season. Morphology of the lesions also considerably helps in arriving at a diagnosis; for example, hydroa vacciniforme (HV) yields varioliform scarring lesions. In addition, histopathological study as well as specialized tests such as phototesting, photoprovocation, photopatch test, and certain serological tests may be attempted.
Autoimmune connective tissue diseases (AICTDs) and inherited disorders require imaging studies and more sophisticated investigations, which will not be discussed further. However, when in doubt certain screening tests such as serum anti-nuclear antibody (ANA), global and urine porphyrins can rule out these conditions.
Morphology of the lesions
PMLE
Lesions are predominantly seen on extensor forearms, whereas the sides and nape of neck and other exposed areas may also be involved. Lesions are often itchy and varying morphologies exist: Eczematous, shiny pinpoint lichenoid, hypopigmented, macular, papular, vesicular, erythema multiforme, or plaque-like forms. Differential diagnoses include other photodermatoses, light-exacerbated atopic eczema, pityriasis versicolor, and lichenoid dermatitis. Although lesions are polymorphic in general, in a given patient, they tend to be monomorphic in nature.
HV
This is rare in Indian subcontinent, and is seen predominantly in boys, as papules topped with vesicles heal with varioliform scarring, and usually resolved by adulthood in most cases. It has to be differentiated from HV-like lymphoproliferative disorder, which is a rare Epstein–Barr virus-(EBV) related lymphoproliferative photodermatoses.[2]
Erythropoietic protoporphyria (EPP)
It presents with an erythematous, edematous rash with a severe burning sensation or even acute pain on sun exposed areas.[3]
Actinic prurigo (AP)
Papules topped with vesicles or crusts are seen on sun-exposed areas. Cheilitis and conjunctivitis are often present.
Chronic actinic dermatitis (CAD)
Eczematous, infiltrated, and lichenified plaques are seen on photoexposed areas, especially the face, with typical summer worsening. It has to be differentiated from systemic photoallergic dermatitis, cutaneous T-cell lymphoma and chronic atopic dermatitis.
Solar urticaria (SU)
Wheals are seen and must be differentiated clinically from EPP (which may present with diffuse swellings with petechia rather than wheals, and pain is more than pruritus), drug- induced phototoxic reaction with urticaria, acute or chronic urticaria due to other causes and urticated PMLE.[4]
AICTD
Systemic lupus erythematosus (SLE), discoid lupus erythematosus, subacute lupus erythematosus (SCLE) dermatomyositis, and photosensitive genodermatoses have characteristic clinical features that are well known and are easily recognizable by proper history and clinical examination.
Inherited disorders of photosensitivity such as xeroderma pigmentosum and trichothiodystrophy have characteristic morphologies and serological and genetic abnormalities, which are out of the scope of this article.
Histopathology
This is useful in differentiating many conditions, especially PMLE from SCLE and actinic reticuloid (AR) from mycosis fungoides. A study by Megahed and Schaller has described the utility of histopathology in various photodermatoses.[5] They concluded that histopathology does not have pathognomonic diagnostic features for most conditions, though diagnostic pointers are available for PMLE (prominent papillary edema) and phototoxic dermatitis (sunburn cells). For example, SU cannot be differentiated from urticaria and HV can be mistaken for bullous phototoxic contact dermatitis, bullous irritant contact dermatitis, or hand,foot mouth disease. Most other conditions have non-specific histopathology.[5] Table 2 denotes the utility of histopathology in the diagnosis of various photodermatoses.[1,3,5,6]
| Photodermatoses | Histopathological finding |
|---|---|
| PMLE | Prominent papillary dermal edema with dense perivascular lymphocytic infiltrate, and superficial and deep. |
| Actinic prurigo | Epidermal spongiosis, dermal perivascular infiltrate, later irregular hyperplasia of epidermis, and focal papillary dermal fibrosis. |
| Actinic cheilitis | Dense lymphocytic infiltrate with well-formed lymphoid follicles. |
| Hydroa vacciniforme | Early lesions – epidermal spongiosis, ballooning degeneration of keratinocytes, later multilocular intraepidermal bullae with fibrin, and reticulate necrosis of keratinocytes. Dermis shows perivascular inflammatory infiltrate. |
| CAD | Highly variable features. Epidermis: spongiosis, lymphocyte exocytosis, occasional atypical mononuclear cells resembling the Pautrier microabscess mimicking CTCL. Occasional parakeratosis. Dermis: superficial and deep perivascular, lymphohistiocytic infiltrate, occasional eosinophils, and plasma cells. In severe cases, infiltrate extends to subcutis. |
| Phototoxicity | Ballooning of keratinocytes with scattered apoptotic keratinocytes and necrotic keratinocytes with pyknotic nuclei and eosinophilic cytoplasm (sunburn cells) in upper third of epidermis, variable spongiosis, and mild to moderate superficial dermal inflammatory cell infiltrate. If severe, epidermal necrosis occurs. |
| Photoallergy | Epidermal spongiosis, acanthosis, superficial perivascular infiltrate with lymphocytes, and eosinophils. |
| EPP | Acute: vacuolization of epidermal endothelial cell lysis within dermal blood vessels. Older lesions: waxy scarring, PAS positive deposits around blood vessels. Ultrastructure finding: capillary basement membrane thickening and degeneration. |
| Lupus erythematosus | Epidermis shows interface dermatitis with vacuolar degeneration of basal cells. In DLE, there is prominent follicular plugging. PAS-positive thickened, occasionally tortuous basement membrane zone. Dermis shows perivascular, periadnexal lymphohistiocytic infiltrate. Mucin deposition in dermis. |
PMLE: Polymorphous light eruption, CAD: Chronic actinic dermatitis, EPP: Erythropoietic protoporphyria, CTCL: Cutaneous T-cell lymphoma, PAS: Periodic acid-Schiff, DLE: Discoid lupus erythematosus.
Photo-testing
This is primarily used in the diagnosis of immunological photodermatoses such as CAD, SU, and PMLE. This helps in determining the action spectrum of the condition so as to avoid the implicated spectrum and to plan the treatment.[7]
Procedure
The patient should avoid topical and systemic steroids and other immunosuppressants for at least two weeks and antihistamines for two days prior to the test. A photo opaque template with multiple 2 × 2 cm windows is placed on the shaved, uninvolved back of the patient. Upper arm or forearm can also be used. The skin is then exposed to increasing dose of UVA, ultraviolet B (UVB), or visible light (VL).
For UVA testing, the following may be used as a source of UVA: High output fluorescent black light used for psoralen with ultraviolet A (PUVA) therapy, PUVA chamber with the patient in the center, or metal halide lamps (UVASUN 320– 400 nm). The doses given are 3, 6, 12, and 18 J/cm2.
For UVB testing, broadband UVB lamps or fluorescent lamps (Philips TL 20 W/12, 285–350 nm) are used and the UVB dose used starts from 6 mJ/cm2, proceeding to 12, 24, 36, 48, 72, 96, and 108 mJ/cm2. A solar simulator was used earlier for calculating the minimal erythema dose (MED) [Figures 1 and 2].

- MED testing using solar simulator. MED: Minimal erythema dose.
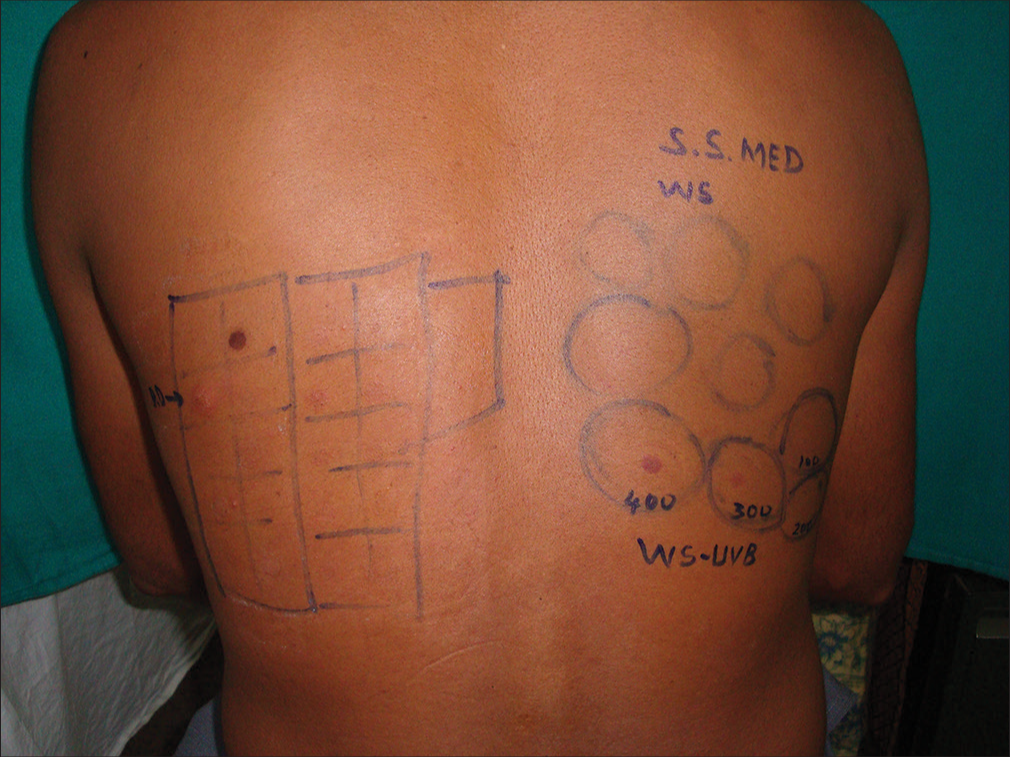
- MED obtained using solar simulator. MED: Minimal erythema dose. SS: Solar simulator, WS: Whole spectrum.
A solarimeter or a slide projector can be used for VL testing and has to be placed 30 cm away from the skin surface. To avoid heat, a water filter is suggested to be placed in front of the light source. The exposure times are 15, 30, 45, and 60 minutes. Elimination of heat rays using water bath will negate the chance of developing heat-induced urticaria.
An immediate reading at 20 min rules out SU. The lowest irradiation that induces wheal is defined as the minimal urticaria dose (MUD). After 24 h, MED for UVA, UVB, or VL is calculated. MED is defined as the dose that induces just perceptible erythema covering the entire irradiated surface [Figure 3].
- MED to UVB at 30J and to UVA at 300J in CAD. MED: Minimal erythema dose, CAD: Chronic actinic dermatitis, UVA: Ultraviolet A, UVB: Ultraviolet B.
Interpretation
MED varies based on Fitzpatrick’s skin types, with darker skins having larger values. There is a lack of Indian studies evaluating MED for UVB, though, for NB-UVB, the average MED calculated was 61.5 ± 17.25 J/cm by Pai et al.[8]
For UVA testing, any response in the range of 3–18 mJ/cm2 is considered to be abnormal; for VL, any urticaria or erythematous response at any time would be considered a positive response.[7]
The expected results for various photodermatoses are as follows: [1,7]
PMLE: Artificial induction of the lesion is difficult, as repeated daily exposures rather than a single photo-provocation causes the lesions. Lesions have been induced by solar simulators, UVA, UVB, as well to VL.[6] MED to UVA and UVB may be normal or reduced whereas that to VL is normal.
CAD: MED to UVA and UVB is reduced whereas, it may be normal or reduced for VL.
SU: Wheals may occur with UVA, UVB, and VL.
AP: MED to UVA is reduced and UVB is normal or reduced, VL is normal or reduced. A study by Crouch et al. found reduction in MED in up to 60% cases.[9]
HV: MED to UVA is reduced with the action spectrum being at 320–390 nm, UVB is mostly normal, and VL is normal. Phototoxicity and photoallergy: MED to UVA is reduced, whereas MED of UVB and VL is normal.
EPP: It presents with burning, swelling, erythema, occasional wheals, and vesicles to VL.[7]
Occasionally in SU, when one action spectrum causes wheal formation, another action spectrum of a longer wavelength is found to inhibit the wheal, leading to the “double action spectrum” phenomenon. Sometimes, an augmentation action spectrum is encountered.
Cutaneous lupus erythematosus (CLE)
A study by Lokitz et al. failed to induce lesions in nine patients with SCLE either by UVA or UVB, and they concluded that the possible role of rays outside this spectrum has to be explored in CLE.[10]
Phototesting with UVA and UVB was also used to study the photoprotective effect of sunscreen in CLE in 25 patients by Kuhn et al. He found that in all 25 patients no lesions were induced on the area where sunscreen was applied, whereas lesions could be induced in 16 patients where it was not applied, and in 14 patients in vehicle applied areas, by irradiation.[11]
A retrospective study on photo testing by Pralong et al. which included 100 cases led to a definitive diagnosis in 60%, maximum cases being PMLE in 20 and photocontact dermatitis in 14. The allergens were nonsteroidal anti-inflammatory drugs (NSAIDs), sunscreens, and fragrances.[12] Tisack et al. have come up with recommendations for various methods of phototesting.[13]
Provocative testing
In conditions such as PMLE, HV, or EPP, lesions may be induced by provocative testing. This is especially effective where MED is suspected to be normal. The same site is exposed to UVA, UVB, or VL for three–four consecutive days at 80% of MED with a subsequent 10%–20% increase.[7] Lesions occur within 24 hours and biopsy may be done if required. This method is not very logistical or popular as the patient has to visit the hospital or clinic daily for 4–5 days. Photoprovocation is positive in 60–70% of cases of AP.[14]
Phototesting and photoprovocative testing have also been used to establish the protective role of sunscreens in photodermatoses.[11]
Photopatch testing
This is used to identify allergens or photoallergens that can exacerbate photodermatoses in suspected cases of photoallergic contact dermatitis (PACD) and is also often positive in CAD.[15] The methodology is the same as patch testing, with allergens being applied in duplicate so that one set can be irradiated by UVA light. Various preformed series of photoallergens are available.[16] There is no Indian series, though European, Scandinavian, and extended European series are available.[17,18]
A photopatch test series ideally contains sunscreens, NSAIDs, cosmetics, and often the patient’s products. In addition to photoallergen series, any suspected product the patient is exposed to, or used by the patient can also be applied in duplicate patches, in sufficient dilution using a suitable substrate after referring to the standard pharmacopeia textbooks.
Method
Duplicate patches of allergens are placed on the uninvolved back on either side. These are covered with opaque material. After 24 or preferably after 48 h, the patch test reading is noted from both sides, and one set is irradiated with UVA light 5 J/cm2 or 10 J/cm2 depending on centers, the other set remains covered. After another 48 h, the patch test reading is again taken from both sides. Seventy-two- and 96-h readings are advisable to look for crescendo and decrescendo patterns of allergy and non-allergy however, are impracticable due to the prolonged nature of the test.[19]
Interpretation
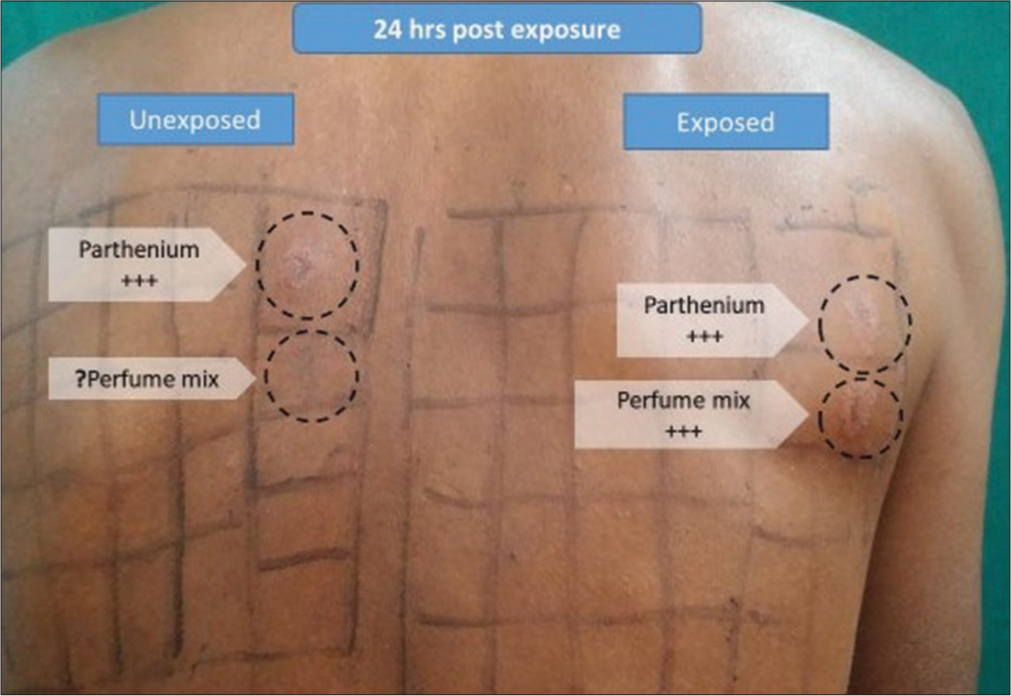
The results are interpreted according to the International Contact Dermatitis Reading Group (ICDRG) criteria as varying degrees of erythema, papules, and vesicles.[20] [Figure 4].
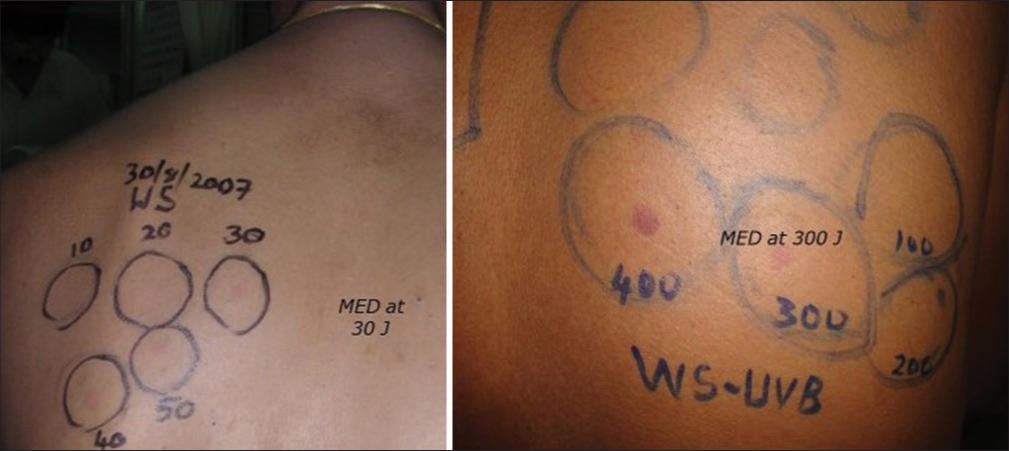
- Photo patch test showing contact allergy to parthenium and photocontact allergy to perfume mix. WS: Whole spectrum, MED: Minimal erythema dose, UVB: Ultraviolet B.
Photopatch test is expected to be positive in photoallergic dermatoses and may occasionally be positive in CAD. There can be photoaggravation as well as photoaugmentation; in photoaggravated reaction, there is contact allergy with photoaggravation whereas photoaugmented reactions have both contact as well as photocontact allergic reactions.[18]
Table 3 shows various common scenarios of photopatch test readings.
| Clinical diagnosis | Patch test | Photo patch test (Irradiated site) |
|---|---|---|
| No allergy | −ve | −ve |
| ACD | + | + |
| PACD | −ve | + |
| Both ACD and PACD | + | ++ |
ACD: Allergic contact dermatitis, PACD: Photoallergic contact dermatitis
Rai and Thomas have done photopatch testing in 35 photodermatitis patients, using 14 J/cm2 UVA irradiation, and found 51% photopatch test positivity with both PACD and photoaggravation to parthenium.[21] A European multicentric study yielded 19.4% positivity in 200 subjects, mostly to topical NSAIDs (ketoprofen and etofenamate) and organic UV absorbers (octocrylene, benzophenone-3, and butyl methoxydibenzoylmethane). Allergic contact dermatitis (ACD) was seen in 5% cases.[22] Data from 1129 patients from 45 centers spanning five years concluded that NSAIDs, sunscreens, disinfectants, fragrances, and phenothiazines caused photoallergy, whereas phototoxicity was attributed to chemicals such as promethazine, chlorpromazine, fenticlor, balsam of Peru, wood tar, and perfumes. However, many reactions lacked relevance for the patients.[23]
Miscellaneous tests
Serum immunoglobulin E is elevated in up to 50% of cases of severe AP.
Occasional Sezary cells in peripheral smear may be seen in CAD.
Other serological tests: ANA, anti-Ro, anti-La, HIV, especially in younger individuals to rule out autoimmune connective tissue diseases.[24]
Tzaneva et al. showed that although, up to 11.7% of PMLE patients show ANA positivity, none progress to SLE;[25] hence, ANA testing need not be performed unless there is a strong suspicion of autoimmune connective tissue disease otherwise.
Porphyrin levels can either confirm or rule out porphyrias. Microcytic anemia with elevated iron levels, cholelithiasis, and cholestasis may also be seen in porphyrias.[1]
Immunohistochemistry shows T-cell cytotoxic molecules and monoclonality of T-cell receptor genes in Epstein Barr-related HV like reactions. This may be used to differentiate from idiopathic HV. TCR gene rearrangements, Ki-67 index, and lactate dehydrogenase levels may also be used.[26,27]
Targeted next-generation sequencing tests, DNA repair function tests using cultured fibroblasts can be used to investigate Xeroderma pigmetosum types and variants, and for atypical cases.[28]
Polarized light microscopy of hair reveals the tiger tail pattern of altering light and dark bands in trichothiodystrophy. Common photodermatoses and investigations are shown in Table 4.
| Condition | Phototesting | Photoprovocation | Photopatch test | Other associations |
|---|---|---|---|---|
| PMLE | Mostly negative | May be positive | Negative | ANA+ve in approximately 13% |
| AP | Negative | HLA-DRB10407+ve (60%) | ||
| HV | Decreased MED to UVA | Negative | Rule out HV like lymphoproliferative disease | |
| SU | Wheals in 20 min with UVA, B or VL | Negative | Circulating IgE | |
| Photoallergic dermatitis | Decreased UVA/UVB | May be positive | Positive | ACD, Photoaugmentation |
| CAD | Decreased MED to UVA, B, VL | Positive | May be positive | Occasional atypical lymphocytes |
| Porphyrias | Mostly negative | Negative | Serum porphyrins |
PMLE: Polymorphous light eruption, AP: Actinic prurigo, SU: Solar urticaria, HV: Hydroa vacciniforme, CAD: Chronic actinic dermatitis, MED: Minimal erythema dose, UVA: Ultraviolet A, UVB: Ultraviolet B, VL: Visible light, ACD: Allergic contact dermatitis, IgE: Immunoglobulin E, ANA: Anti-nuclear antibody, HLA-DRB1 Human leukocyte antigen class II histocompatibility, D related beta chain.
TREATMENT
General principles
All patients of photodermatoses should follow strict photoprotection by means of clothing, hats, shawls, sunglasses, and avoidance of direct sun exposure. Broad-spectrum sunscreen may be used wherever indicated, provided that there is no photocontact dermatitis. Window films that block UVA and VL to some extent can be used in cars and home window panes. Clothing that is especially suitable to prevent UV light entry is available, with a specific ultraviolet protection factor (UPF) protection factor mentioned; higher the UPF, better the protection. Clothing treated with broad spectrum UV absorbers is also available.[29] In case of sunburn such as reactions, cold compresses, antihistamines, analgesics, and bland emollients are to be used. Avoidance of all known allergens, photoallergens, and photosensitizing drugs is the norm in conditions of photoallergy, phototoxicity, and severe photosensitivity like CAD. Even broad-spectrum sunscreens should preferably be used only after ruling out sunscreen allergy.[1] Topical steroids may be applied to the skin lesions, depending on the area and severity of involvement, and may be tapered and stopped based on the response. Some commonly available topical steroids in increasing order of potency are hydrocortisone acetate 1%, desonide 0.05%, hydrocortisone butyrate 0.1%, mometasone furoate 0.1%, fluticasone propionate 0.05%, betamethasone valerate 0.1%, betamethasone dipropionate 0.05%, clobetasol propionate 0.05%, halobetasol propionate 0.05% available as lotion, cream, and ointment.[30] Topical calcineurin inhibitors such as tacrolimus 0.01% ointment or lotion can also be used.
PMLE
For extensive and severe PMLE, short course of oral steroids, or steroid-sparing agents like hydroxychloroquine (HCQ) sulfate (200–300 mg/day), azathioprine (0.8–2.5 mg/kg/day), cyclosporine (3–5 mg/kg/day), or even thalidomide has been tried.[3,6] Topical antioxidants have been evaluated and found to be effective in UVA and UVB-mediated photodermatoses by reducing MED as well as minimal phototoxic dose. Topical tocopherol has been used in PMLE.[31] Oral polypodium leucotomos extract 240 mg twice daily started 15 days before sun exposure, and continued throughout is reported to be beneficial by Caccialanza et al.[32] Afamelanotide, an alpha melanocyte-stimulating hormone analog, is a promising drug that has completed phase III clinical trials in PMLE, and acts via skin pigmentation, DNA repair, and antioxidant repair. It is available as a 20 mg subcutaneous implant.[33] In CAD, the patient should avoid contact and photocontact allergens as well as potent photosensitizers. Sunscreen allergy should be ruled out. Azathioprine, cyclosporine, mycophenolate mofetil (MMF), and even tofacitinib have been tried in CAD.[34] Bhari and Gupta have attempted overnight application of 0.1% tacrolimus under occlusion in resistant CAD.[35] Photoallergic reactions can be treated similar to ACD with topical or oral steroids and steroid sparing agents. If every severe and extensive, a short course of oral steroids, approximately 0.25–0.5 mg/kg equivalent of prednisolone may be required, with due precautions. Polypodium leucotomos, pentoxifylline, cyclosporine, azathioprine in immunosuppressive doses, thalidomide 50–100 mg/day, and even Dupilumab have been tried in CAD and resistant AP cases.[36] Eickstaedt et al. reported clearance of recalcitrant AP with Dupilumab.[37]
SU
Non-sedative antihistamines along with photoprotection form the first-line treatment followed by phototherapy. Omalizumab 300 mg s/c at 4 weeks interval is effective, especially in refractory cases of SU.[38] Prophylactic use of antihistamines 1 h before sun exposure can prevent lesions. Cimetidine, doxepin, leukotriene antagonists, intravenous immunoglobulin (400 mg/day for 5 days), and cyclosporine (3–5 mg/kg/day) have also been tried.[3]
AP is often resistant to treat even with phototherapy, and if so, thalidomide is the treatment of choice and can be started at 100–200 mg/day and later tapered to 50 mg once in 2–3 days.[3,4]
Porphyrias
Cholestyramine can bind to, and excrete protoporphyrins in feces. Oral beta-carotene, activated charcoal, colestipol, cimetidine, and plasmapheresis are other modalities. For porphyria cutanea tarda, avoid alcohol, smoking, and added estrogens. Phlebotomy, which reduces iron-mediated uroporphyrinogen decarboxylase inhibition and low-dose HCQ (100 mg twice a week), is also tried.
Afamelanotide that stimulates melanin production and increases the light tolerance, is considered the currently most effective treatment. In porphyrias, it is given as a 60 mg s/c depot injection every 60 days.[39] Liver and bone marrow transplantation are end-stage options.
Long-term oral retinoids (isotretinoin and acitretin) and topical imiquimod are tried in Xeroderma pigmentosum, to prevent cutaneous malignancy.
Sunscreens with T4 endonuclease V bacterial DNA repair enzyme or with photolyases can reduce development of actinic keratoses and cutaneous malignancies, but are not in wide use.[40] Regular cutaneous, neurological, and ophthalmologic checkups should be carried out, and any malignancies should be removed at the earliest.
SUNSCREEN PRODUCTS
Sunscreens can be systemic or topical, and topical sunscreens can further be physical or chemical sunscreen or a combination or both.
Sunscreen efficacy is measured in many ways including sun protection factor, substantivity, immune protection factor, and various regional guidelines such as Japanese, Australian/New Zealand, European Union Guidelines, and Boots star rating system. An ideal sunscreen should have broad spectrum coverage, high substantivity, be photostable, cosmetically elegant, non-allergenic and non-irritant, non-comedogenic, and most importantly, affordable for long-term use. Newer sunscreens are under research and development, with broad UV spectrum filters for high-energy visible radiation (HEVR), namely, TriAsorB, a low– molecular-weight HEVR filter which also protects against oxidative DNA damage, broad spectrum photostable filters such as Parsol® Max, DSM, and Bis (diethylamino hydroxybenzoyl) piperazine are in the offing. Cooling filters containing hydrogels with hyaluronic acid and tannic acid with broad-spectrum UV protection which maintains excellent skin adhesion and has antioxidant as well as skin cooling properties are being developed.
Nanocrystal technology as well as methacrylate polymers and netlock technology have stabilized the sunscreens, thereby reducing penetration within the skin.
Natural products like mycosporine-like amino acids (MAAs) isolated from fungi and marine animals, flavonoids like Lignin, Scytonemin pigment from cyanobacteria, polyphenols like Silymarin, and topical as well as oral Polypodium leucotomos are being considered as future “green” (environmentally acceptable) sunscreen ingredients.[41]
Sunscreens should be applied in adequate amount, at least 20–30 min before sun exposure, and reapplied every 3 h if the exposure is prolonged. The concentration on the body should be 2 mg/cm2 which comes to about 3 mL for face and neck, and arms, respectively, and 6 mL for each leg, chest, and back. (“the teaspoon rule;” 1 tsp = 5 mL).[6]
PHOTOTHERAPY
Mechanism
Hardening induced by phototherapy results in downregulating the immune response to neoantigens formed during the disease process.[42] There is also disruption in antigen presentation and secretion of inhibitory cytokines.[43] In SU, it works by downregulation of immunoglobulin E production and inhibition of mast cell degranulation.[8] Photodermatoses managed by phototherapy include PMLE, SU, AP,[44] chronic actinic dermatitis, porphyria,[45] and HV.[46] Contra-indications to phototherapy include patients with SLE, Xeroderma pigmentosum.
Protocol
Patients should be explained the procedure, the mechanism, cost, need for multiple sessions, and the possible side effects, including tanning. If possible, proceed with MED testing to know the degree of photosensitivity.
In patients with severe photosensitivity, especially in CAD, some authors recommend a short course of oral steroids for 8–10 days, at approximately 0.6–0.8 mg/kg body weight equivalent of prednisolone, from the day before initiation of phototherapy.[8]
For those with normal MED, starting dose is based on the skin type, or the protocol of the respective phototherapy unit, and subsequent increment is 10–15%/session, with 2–3 sessions/week.
Photo therapy
UVA1 is not available freely in India. However, if attempted, MUD for UVA1 should be elicited, and therapy should be started with 50% of the same. If not, start with 10 J/cm2 with increments of 5–10 J/cm2 until a total dose of 20 J/cm2. Only photo-exposed sites may be treated, so as to minimize the chance of anaphylaxis.
Narrow-band ultraviolet B (NB-UVB) is currently the phototherapy of choice, due to its lesser side effects and ease of administration. The starting dose varies depending on skin type, but can be anywhere between 200 and 300 mJ/cm2 for Indian skin. Treatments are spaced at two to three –3/week for a total of 15 sessions. Patients may then be advised to expose to 20–30 min of cumulative weekly midday sun exposure to maintain the hardening PUVA therapy has been used for CAD, but at a lower dose of 0.5 J/cm2, with 2–3 treatments/week to a total of 15–20 treatments.
In SU, UVA1, PUVA, or NB-UVB may be used.[8] Phototherapy has also been successfully tried in AP, HV, and EPP.[8]
PMLE
Phototherapy is well known to induce prophylactic protection in PMLE. Aslam et al. evaluated the effect of desensitization using phototherapy in 79 patients and found that 91% patients found it to be effective.[47]
CAD
A pilot study on UVA rush hardening in six patients with CAD was conducted by Wang et al., in which patients were exposed to multiple UVA sessions at 1-h intervals for 4–5 days, and subsequent maintenance doses at 1–2 weeks, and this was found to be effective.[48]
SU
There are various studies that have found good outcomes with phototherapy using NB-UVB[49,50] as it is more widely available.
CONCLUSION
To conclude, though clinical acumen has a great impact in diagnosis of photodermatoses, accurate diagnosis of the inducing rays can be done only by phototesting and MED determination. The cornerstones of treatment of photodermatoses include photoprotection, topical and systemic immunosuppression and in selected cases, prophylactic phototherapy for idiopathic photodermatoses, as well as photoaggravated dermatoses.
Points to note
Most photodermatoses are in the UVA spectrum.
UVB-responsive dermatoses are more easily prevented, as UVB does not penetrate glass, and broad-spectrum sunscreen is helpful.
Even potent UVA sunscreen may not fully prevent PMLE if there is a very low UVA threshold.
Sensible, general sun protection behavior should be reiterated to the patient at every visit.
Sunscreens are not protective in the VL range; hence, conditions like EPP or SU may require physical protection with clothes, cap, and shawl.
Rarely, even fluorescent lamps can exacerbate CAD; then, LED lamps are a safer alternative.
Water, snow,and sand can reflect sunlight, hence extra caution to be taken. Tanning beds to be avoided.
Vitamin D supplementation is essential in those on prolonged photoprotection.
Phototherapy is used diagnostically as well as therapeutically; NB-UVB is the most widely used
Biological therapy is evolving with molecules like dupilumab, omalizumab being explored in certain indications.
Promising drugs like afamelanotide are under consideration.
Acknowledgment
The author would like to thank Prof. Sathish Pai B for clinical photographs.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The author certifies that she has obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Photosensitivity disorders (photodermatoses) Clinical manifestations, diagnosis, and treatment Netherlands: Wolters Kluwer; Available from: https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment [Last accessed on 2023 Nov 29]
- [Google Scholar]
- Photodermatoses: What's new. Curr Opin Pediatr. 2022;34:374-80.
- [CrossRef] [PubMed] [Google Scholar]
- Recent developments in the diagnosis and management of photosensitive disorders. Am J Clin Dermatol. 2018;19:707-31.
- [CrossRef] [PubMed] [Google Scholar]
- Photodermatoses: Diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-41.
- [CrossRef] [Google Scholar]
- Histopathologie der photodermatoses [Histopathology of photodermatoses] Hautarzt. 2006;57:1083-8.
- [CrossRef] [PubMed] [Google Scholar]
- Photodermatology and photodermatoses In: Sachchidanand S, ed. IADVL textbook of dermatology (5th ed). Maharashtra: Bhalani Publishing House; 2022. p. :984-1030.
- [Google Scholar]
- Phototherapy in the evaluation and management of photodermatoses. Dermatol Clin. 2020;38:71-7.
- [CrossRef] [PubMed] [Google Scholar]
- MED estimation for narrow band UV-B on type IV and type V skin in India. Indian J Dermatol Venereol Leprol. 2002;3:140-1.
- [Google Scholar]
- Actinic prurigo: A retrospective analysis of 21 cases referred to an Australian photobiology clinic. Australas J Dermatol. 2002;43:128.
- [CrossRef] [PubMed] [Google Scholar]
- Failure of physiologic doses of pure UVA or UVB to induce lesions in photosensitive cutaneous lupus erythematosus: Implications for phototesting. Photodermatol Photoimmunol Photomed. 2006;22:290-6.
- [CrossRef] [PubMed] [Google Scholar]
- Photoprotective effects of a broad-spectrum sunscreen in ultraviolet-induced cutaneous lupus erythematosus: A randomized, vehicle-controlled, double-blind study. J Am Acad Dermatol. 2011;64:37-48.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of phototesting in the diagnosis of photodermatoses: Retrospective study of 100 cases. Photodermatol Photoimmunol Photomed. 2022;38:99-103.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for reporting methods in phototesting studies. Photochem Photobiol. 2022;98:130-1.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of the common idiopathic photodermatoses. Australas J Dermatol. 2003;44:90-6.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic actinic dermatitis: An analysis at a single institution over 25 years. Dermatitis. 2011;22:147-54.
- [CrossRef] [PubMed] [Google Scholar]
- Photopatch test baseline series In: Mackay IR, Rose NR, Ledford DK, Lockey RF, eds. Encyclopedia of medical immunology. New York, NY: Springer; 2014.
- [CrossRef] [Google Scholar]
- Photopatch testing with an extended series of photoallergens: A 5-year study. Contact Dermatitis. 2009;60:325-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of photopatch test allergens for Indian patients of photodermatitis: Preliminary results. Indian J Dermatol Venereol Leprol. 2011;77:148-55.
- [CrossRef] [PubMed] [Google Scholar]
- Photopatch testing In: Lachapelle JM, Maibach H, eds. Patch testing and prick testing. Cham: Springer; 2020.
- [CrossRef] [Google Scholar]
- Patch testing methodology In: Lachapelle JM, Maibach H, eds. Patch testing and prick testing. Cham: Springer; 2020.
- [CrossRef] [Google Scholar]
- Photopatch and UV-irradiated patch testing in photosensitive dermatitis. Indian Dermatol Online J. 2016;7:12-6.
- [CrossRef] [PubMed] [Google Scholar]
- A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-9.
- [CrossRef] [PubMed] [Google Scholar]
- Photopatch testing: The 5-year experience of the German, Austrian, and Swiss Photopatch Test Group. J Am Acad Dermatol. 1991;25:59-68.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of serum IgE levels and clinical manifestations in patients with actinic prurigo. An Bras Dermatol. 2016;91:23-6.
- [CrossRef] [PubMed] [Google Scholar]
- Antinuclear antibodies in patients with polymorphic light eruption: A long-term follow-up study. Br J Dermatol. 2008;158:1050-4.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical hydroa vacciniforme-like Epstein-Barr virus associated T/NK-cell lymphoproliferative disorder. Am J Dermatopathol. 2012;34:e119-24.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like lymphoproliferative disorder: A study of clinicopathology and whole-exome sequencing in Chinese patients. J Dermatol Sci. 2020;99:128-34.
- [CrossRef] [PubMed] [Google Scholar]
- Uncommon nucleotide excision repair phenotypes revealed by targeted high-throughput sequencing. Orphanet J Rare Dis. 2016;22:26.
- [CrossRef] [PubMed] [Google Scholar]
- The Merck manual of diagnosis and therapy (19th ed). United States: Merck Sharp and Dohme Corporation; 2011.
- [Google Scholar]
- Protective effects of topical antioxidants in humans. Curr Probl Dermatol. 2001;29:157-64.
- [CrossRef] [PubMed] [Google Scholar]
- Photoprotective activity of oral Polypodium leucotomos extract in 25 patients with idiopathic photodermatoses. Photodermatol Photoimmunol Photomed. 2007;23:46-7.
- [CrossRef] [PubMed] [Google Scholar]
- Afamelanotide: An orphan drug with potential for broad dermatologic applications. J Drugs Dermatol. 2021;20:290-4.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib in resistant chronic actinic dermatitis: A case series. Dermatol Ther. 2022;35:e15467.
- [CrossRef] [Google Scholar]
- Tacrolimus 0.1% ointment applied under occlusion using cling film clears chronic actinic dermatitis resistant to systemic treatment. Int J Dermatol. 2017;56:e139-41.
- [CrossRef] [Google Scholar]
- Clearance of chronic actinic dermatitis with dupilumab therapy in Chinese patients: A case series. Front Med (Lausanne). 2022;9:803692.
- [CrossRef] [PubMed] [Google Scholar]
- Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical response and long-term follow-up of 20 patients with refractory solar urticaria under treatment with omalizumab. J Am Acad Dermatol. 2023;88:1110-1.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetics and pharmacodynamics of afamelanotide and its clinical use in treating dermatologic disorders. Clin Pharmacokinet. 2017;56:815-23.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive long-term effects of a topical film-forming medical device with ultra-high UV protection filters and DNA repair enzyme in xeroderma pigmentosum: A retrospective study of eight cases. Case Rep Dermatol. 2014;6:222-6.
- [CrossRef] [PubMed] [Google Scholar]
- New developments in sunscreens. Photochem Photobiol Sci. 2023;22:2473-82.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphous light eruption: Clinic aspects and pathogenesis. Dermatol Clin. 2014;32:315-34.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of phototherapy and photochemotherapy for photodermatoses. Dermatol Ther. 2003;16:23-7.
- [CrossRef] [PubMed] [Google Scholar]
- Narrowband ultraviolet B phototherapy in erythropoietic protoporphyria: Case series. Br J Dermatol. 2014;170:987-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme: A clinical and follow-up study of 17 cases. J Am Acad Dermatol. 2000;42:208-13.
- [CrossRef] [PubMed] [Google Scholar]
- Actinic prurigo: Limited effect of PUVA. Br J Dermatol. 1997;136:972-3.
- [CrossRef] [PubMed] [Google Scholar]
- Phototherapy and photochemotherapy for polymorphic light eruption desensitization: A five-year case series review from a university teaching hospital. Photodermatol Photoimmunol Photomed. 2017;33:225-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraviolet A rush hardening for chronic actinic dermatitis: Pilot treatment outcomes. J Dermatol. 2021;48:385-8.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of light tolerance using narrowband UV-B in solar urticaria. Actas Dermosifiliogr (Engl Ed). 2018;109:888-92.
- [CrossRef] [PubMed] [Google Scholar]
- Narrowband ultraviolet B phototherapy is a suitable treatment option for solar urticaria. J Am Acad Dermatol. 2012;67:e5-9.
- [CrossRef] [PubMed] [Google Scholar]






