Translate this page into:
Ambroxol-induced leukocytoclastic vasculitis: A case report and literature review
*Corresponding author: Ankit Bhardwaj, Department of Pharmacology, University College of Medical Sciences, Delhi, India. drankitbhardwaj25@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhardwaj A, Ghadlinge M. Ambroxol-induced leukocytoclastic vasculitis: A case report and literature review. Indian J Skin Allergy. 2024;3:125-7. doi: 10.25259/IJSA_37_2023
Abstract
Cutaneous leukocytoclastic vasculitis (LCV) that primarily affects the skin, can be idiopathic or linked to infections, autoimmune diseases, neoplasms, and medications. An immune complex-mediated vasculitis of the dermal capillaries and venules is the histological hallmark of LCV, a small vessel vasculitis. This case report describes a 36-year previously healthy man who was presented to the outpatient department (OPD) with complaints of edema and development of numerous reddish-purple patches (caused by petechiae) after intake of cough syrup containing ambroxol. Ambroxol affects human monocytic cells THP-1, and prevents the generation of platelet-derived growth factor (PDGF)-AB that is produced by lipopolysaccharide (LPS) by inhibiting the expression of PDGF-A messenger Ribonucleic acid (RNA). An extracellular signal-regulated kinase (ERK) on p44/42 is activated by LPS, and ambroxol reduces this activation. In addition, 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD 98059), a Mitogen-Activated Protein Kinase Inhibitors (MEK), MEK-1-specific inhibitor, prevents p44/42 ERK activation and PDGF synthesis brought on by LPS. Megakaryocytes, erythrocytes, leukocytes, and their progenitors are stimulated to multiply by PDGF to the numerous endogenous growth factors that mesenchymal stem and stromal cells secrete. Ambroxol reduces the number of megakaryocytic progenitors and boosts apoptosis by inhibiting the production of PDGF and related signaling pathways. It is strongly advised that patients must be made more aware of the risks associated with using such over-the-counter medications.
Keywords
Drug-induced leukocytoclastic vasculitis
Over-the-counter drug
Self-medication
Ambroxol
INTRODUCTION
Small dermal capillaries and venules are affected by leukocytoclastic vasculitis (LCV), a vasculitis that can either be idiopathic or linked to an underlying pathology or disease.[1] About 45 people/million worldwide experience biopsy-proven LCV each year. This frequency of LCV incidence is dependent on the underlying etiology. LCV occurs within 1–3 weeks following the start of the alleged medicine, and 90% of cases resolve within a week to a month from the time of onset. Around 50% of LCVs are idiopathic in character and have a self-limited course. LCV has been linked to a number of drugs. Cutaneous drug-induced vasculitis occurs quite commonly, while systemic vasculitis develops only in a minority of patients when treated with a drug over a prolonged period.[2,3] The complete pathogenesis behind drug-induced vasculitis is still unclear. Herein, we report a case of a 36-year-old man with LCV, which occurred after initiation of cough syrup containing ambroxol hydrochloride (HCL). This case report received approval from the Institutional Ethics Committee (IEC) of Atal Bihari Institute of Medical Sciences and Dr Ram Manohar Lohia; approval reference number -(IECHR-2022- 52-139).
CASE REPORT
A 36-year-old man who had previously been in good health presented with complaints of swelling and non-pruritic extensive reddish-purple rashes (1–5 mm) throughout the forearms, abdomen, and thighs. The rash appeared four days following the initiation of ambroxol HCL for cough. The patient refused any history of neurological symptoms, or of melena, of melena, hematochezia, hemoptysis, and hematuria. The patient was a non-smoker with no family history of chronic illnesses or medication allergies. On examination, we found numerous palpable, widespread erythematous maculopapular rashes that ranged from 1 to 5 mm in diameter, which further coalesced into plaques involving the lower trunk, forearm, and thighs [Figure 1]. All blood parameters were in the normal range except the platelet count (17 × 109/L) and immunoglobulin E levels (539 IU/mL). The patient’s reverse transcription polymerase chain reaction for COVID-19 was negative. The throat swab culture did not show any bacterial growth after 48 h of incubation. The serum was negative for antinuclear antibodies, myeloperoxidase antibodies, and proteinase 3 antibodies. Serological tests for hepatitis B, hepatitis C, and HIV were all negative [Figure 2a and Figure 2b]. The microscopic high-power view of the punch biopsy from two distal sites showed LCV of small superficial vessels. Vessel walls were thickened and necrotic due to lymphocytes, neutrophils, and fibrin infiltration around and in superficial vessel walls, swollen vascular endothelium (arrow white), and extravasated erythrocytes (arrow black). The patient was started on tablet deflazacort 30 mg once daily for a week, resulting in full resolution of rashes [Figure 3]. A causality assessment score of 7 (probable) was calculated. No repeat drug challenge test was done in the present case, as our patient clearly remembered that the cutaneous lesions appeared after he ingested ambroxol HCL cough syrup. The patient was educated and advised not to take any ambroxol-containing cough syrup in the future.
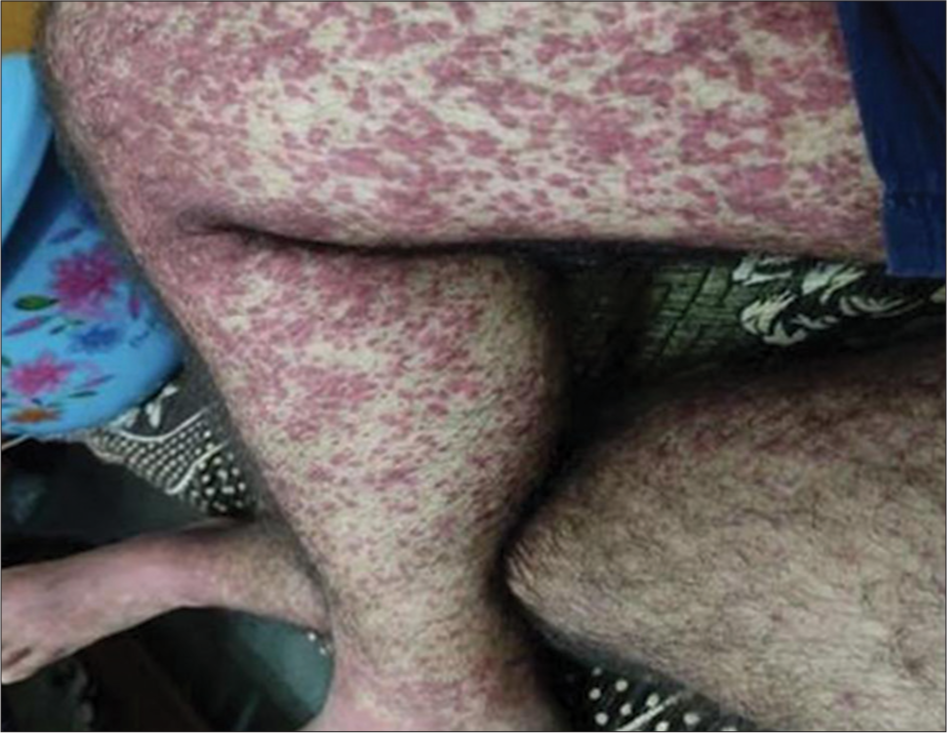
- Palpable extensive erythematous maculopapular purpura (1–5) mm in diameter which further coalesced into plaques involving the lower trunk, forearm, and thighs.
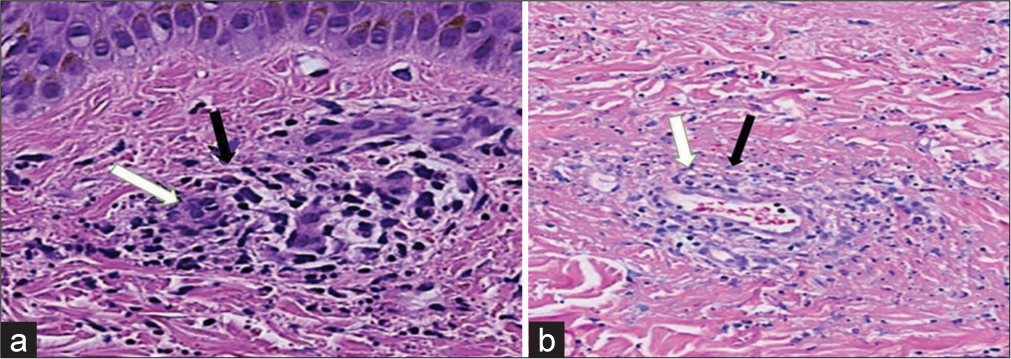
- (a and b) the microscopic high-power view of punch biopsy from two distal sites shows infiltration of the lymphocytes and neutrophils around and in superficial vessel walls, swollen vascular endothelium (arrow white), and extravasated erythrocytes (arrow black).
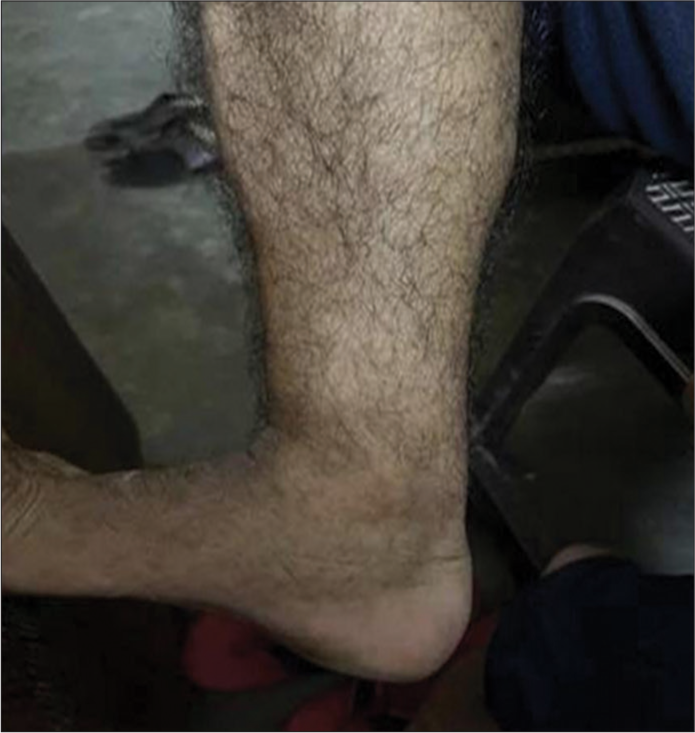
- Post-treatment full resolution of rashes.
DISCUSSION
Ambroxol is a low-molecular-weight chemical causing endothelial damage by forming immunological complexes that get deposited in the cutaneous capillaries. On the other hand, they act simultaneously on human monocytic cells THP-1 and suppress the synthesis of platelet-derived growth factor (PDGF)-AB that is produced by LPS through PDGF-A messenger RNA expression. An extracellular signal-regulated kinase (ERK) on p44/42 gets activated by lipopolysaccharide (LPS), and ambroxol reduces this activation. In addition, 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD 98059), a mitogen-activated protein kinase inhibitors (MEK), MEK-specific inhibitor, prevented p44/42 ERK activation and PDGF synthesis caused by lipopolysaccharide (LPS). Megakaryocytes, erythrocytes, leukocytes, and their progenitors are stimulated to multiply by PDGF, thanks to the numerous endogenous growth factors that mesenchymal stem and stromal cells secrete.[4] An earlier study from 2006, validated the safety and effectiveness of ambroxol. Based on a literature review conducted by the China Knowledge Infrastructure (CNKI’s) a total of 52 cases of ambroxol induced adverse drug reactions had been reported from January 2002 to July 2011. Systemic responses made up the majority of the ADRs (32 instances), followed by lesions of the skin and its appendages (10 cases).[5] The total number of these cutaneous ADRs is quite low when compared to the length of the review. The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency has examined the security of medications containing ambroxol. Ambroxol has a risk of hypersensitivity reactions and a potential risk of severe cutaneous adverse reaction (SCARs), according to the PRAC assessment. Following this, the PRAC suggested, including a warning for patients and caregivers to recognize the prodromes of cutaneous responses and to stop using ambroxol right away in the product information leaflets to address the risk of cutaneous adverse reactions. Only two case reports of cutaneous drug eruptions following the use of ambroxol cough syrup to treat a sore throat were found in the literature review. Based on genetic investigations, the presence of Platelet-derived growth factor receptor A (PDGFR-a) has been proven in human megakaryocytes and platelets. Ambroxol reduces the number of megakaryocytic progenitors and boosts apoptosis by inhibiting the production of PDGF and related signaling pathways. This could be the mechanism causing both henoch-schönlein purpura (HSP) and LCV to develop in our patients. LCV is characterized by tiny vessel involvement and palpable and itch or non-itchy lesions on the lower extremity. The same was observed in our patient. Extracutaneous involvement may be present in roughly 30% of people. Only a few case reports of LCV with purpura caused by bromhexine and ambroxol are found in the literature.
CONCLUSION
This case report shows that a handful of reports can only be the tip of the iceberg in ambroxol-induced cutaneous drug reactions. Ambroxol-containing cough syrups are easily available as over-the-counter (OTC) medicines. An OTC drug is a double-edged sword that requires due consideration for patient safety. Measures to increase patients’ awareness regarding the adverse effects related to the use of such OTC drugs are highly recommended
Ethical approval
This case report received approval from the Institutional Ethics Committee (IEC) of Atal Bihari Institute of Medical Sciences and Dr Ram Manohar Lohia; approval reference number - (IECHR-2022- 52-139).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Propylthiouracil-induced autoimmune syndromes: Two distinct clinical presentations with different course and management. Semin Arthritis Rheum. 2006;36:4-9.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of severe allergic reactions with ambroxol and bromhexine-containing medicines considered small Dublin: Health Products Regulatory Authority; 2015.
- [Google Scholar]
- Safety and usage pattern of an over-the-counter ambroxol cough syrup: A community pharmacy-based cohort study. Int J Clin Pharmacol Ther. 2006;44:409-21.
- [CrossRef] [PubMed] [Google Scholar]






