Translate this page into:
Hair depigmentation in a patient treated with pazopanib: A case report and a comprehensive analysis
*Corresponding author: Varun Goyal, Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Institute, Delhi, India. drvarungoyaloncologist@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jain A, Goyal V, Soni S, Narayan S, Redhu P, Mathighatta Shivarudraiah SS, et al. Hair depigmentation in a patient treated with pazopanib: A case report and a comprehensive analysis. Indian J Skin Allergy. 2024;3:71-3. doi: 10.25259/IJSA_52_2023
Abstract
Pazopanib is a multitargeted tyrosine kinase inhibitor used for the treatment of various solid tumor malignancies. Hair color change, particularly hair depigmentation, is a common side effect of pazopanib therapy. However, it usually develops gradually over a span of few months. This article will provide a comprehensive analysis of hair depigmentation in patients treated with pazopanib, focusing on the various aspects of this phenomenon, including its onset, possible causes, and potential implications for therapy success. Multitargeted tyrosine kinase inhibitors (MKIs) including pazopanib are recommended for various malignancies. Hair color changes are known side effects of MKIs. The exact mechanisms behind hair depigmentation due to pazopanib therapy are not yet fully understood. However, researchers believe that several factors may contribute to this phenomenon. We presented a case of a patient with relapse Ewings sarcoma who experienced rapid hair depigmentation during pazopanib therapy.
Keywords
Pazopanib
Multitargeted tyrosine kinase inhibitor
Hair depigmentation
INTRODUCTION TO PAZOPANIB
Pazopanib is a multitargeted tyrosine kinase inhibitor that is primarily used for the treatment of metastatic renal cell carcinoma. It works by inhibiting the growth of new blood vessels in tumors, thereby restricting the supply of oxygen and nutrients needed for their growth. Pazopanib has shown promising results in clinical trials, significantly improving progression-free survival and overall survival rates in patients with advanced renal cell carcinoma.
CASE REPORT
A 28-year-old man was evaluated at our institute with complaint of c/o Hematuria for two weeks. Computed tomography (CT) of the abdomen revealed heterogeneously enhancing, exophytic left renal mass measuring 115 × 96 × 86 mm with the cystic component. The patient underwent left radical nephrectomy with liver mobilization and inferior vena cava thrombectomy and was diagnosed as a round blue cell tumor. Tumor cells showed strong diffuse positive for cluster of differentiation (CD99), negative for wilms tumor protein (WT1), synaptophysin, desmin, leukocyte common antigen (LCA), and paired-box gene 8 (PAX8). Post-operative positron emission tomography computed tomography (PET CT) was s/o metabolically active abdominal lymph node, multiple bilobar hepatic lesions, and pulmonary nodules. Patient was planned for palliative chemotherapy. He received eight cycles of vincristine, adriamycin, and cyclophosphamide alternating with ifosfamide and etoposide (IE)-based chemotherapy.
In view of progression of the disease, the patient was advised for second-line palliative chemotherapy. He received four cycles of topotecan and cyclophosphamide based chemotherapy. Follow-up PET-CT was suggestive of progressive multiple nodules in the lung, liver lesions, and mesenteric deposit. The patient was started on pazopanib 800 mg once daily. He was on regular follow-up in outpatient department. After three months of therapy, the patient gradually noticed that his hair and eyebrows turned white. The hair depigmentation exhibited symmetrical and progressive patchy involvement, impacting both the scalp hair and eyelashes [Figure 1]. There were no accompanying cutaneous manifestations observed. We had discussed with our dermatologist, who advised investigations aiming to understand the sudden hair depigmentation during pazopanib therapy. These investigations include blood tests assessing Vitamin B12, Vitamin D, and thyroid levels, a genetic evaluation exploring potential links to premature graying, and a scalp examination to identify any specific damage or conditions associated with the observed depigmentation.
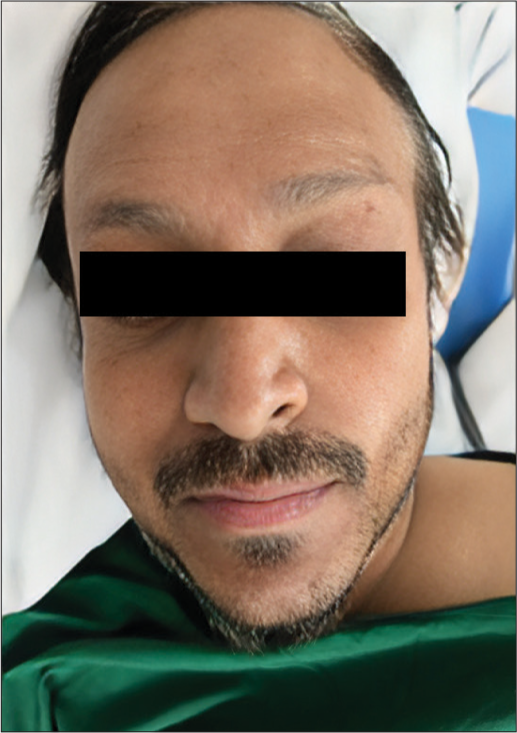
- Patient with hair color changes (depigmentation) on treatment with pazopanib.
The patient was continued on Tab pazopanib for four months. On follow-up PET CT scan multiple new progressing liver, lung nodules were developed. Consequently, the treatment was shifted to irinotecan and temozolomide as the next line of systemic therapy. Unfortunately, after completing three cycles of this therapy, the disease continued to progress. Considering the patient’s overall health condition and the disease’s resistance to treatment, a decision was made, following a multidisciplinary discussion, to transition to supportive care only. Eventually, the patient’s condition deteriorated, and they passed away during the course of their illness.
DISCUSSION
Hair depigmentation, or the loss of hair color, is an intriguing side effect associated with the use of pazopanib, a medication used to treat solid tumor malignancies like metastatic renal cell carcinoma. This side effect typically occurs gradually over several months of therapy, but there have been rare reports of rapid hair and eyebrow depigmentation occurring within weeks of starting pazopanib treatment.
The exact mechanism behind pazopanib-induced hair depigmentation remains unclear. Researchers have proposed several possible explanations based on the understanding of melanogenesis and the effects of pazopanib on cellular signaling pathways.[1] One possible mechanism is the direct inhibition of tyrosinase, an enzyme involved in melanin production. Pazopanib may also disrupt the stem cell factor (SCF)/c-Kit signaling pathway that plays a role in hair pigmentation regeneration.[2]
Hair depigmentation caused by pazopanib therapy may serve as a potential predictor of treatment success. In some cases, patients who experienced rapid hair depigmentation had a favorable response to pazopanib, suggesting a correlation between the severity and rapidity of depigmentation and drug efficacy. However, further research is needed to validate this correlation across different cancer types.
Other tyrosine kinase inhibitors, such as dasatinib and imatinib mesylate, have also been associated with hair depigmentation, although the onset and severity may differ.[3] However, in most cases, hair depigmentation is considered a cosmetic side effect and does not require discontinuation of the medication.[4,5]
Management of hair depigmentation caused by pazopanib or other tyrosine kinase inhibitors focuses on providing support and counseling to patients. This may include discussing potential side effects, encouraging patients to report any changes in hair color or texture, and providing tips for coping, such as using wigs or cosmetic products.[6]
The current knowledge regarding hair depigmentation is limited, and further research is necessary to understand the underlying mechanisms and determine its implications for therapy success [Table 1]. Future studies should focus on molecular mechanisms, biomarker identification, and personalized treatment strategies.
| Author | Year | Patient characteristic | Dosage | Hair depigmentation | Other side effects |
|---|---|---|---|---|---|
| Yun et al.[4] | 2015 | 38-year-old man with metastatic renal cell carcinoma | 800 mg/day | By the fifth month of therapy, nearly all of his scalp hair had turned white | Diarrhea, nausea, vomiting, fatigue, rash, and hypertension |
| Šeparović et al.[5] | 2018 | 62-year-old man with metastatic renal cell carcinoma | 800 mg/day | Rapid overnight hair and eyebrow depigmentation after three weeks of pazopanib therapy | Diarrhea, nausea, vomiting, hand-foot syndrome, hypertension, and hypophosphatemia |
| Harrak et al.[6] | 2021 | 26-year-old male with metastatic renal cell carcinoma | 800 mg/day | hair depigmentation starts two months after the beginning of treatment with pazopanib | diarrhea, hypertension, fatigue, and, above all, hepatic toxicity |
| Hasan et al.[3] | 2023 | 70-year-old Nigerian male with CD 117 positive GIST on Imatinib | 600 mg/day | Three months after starting imatinib mesylate, had hypopigmentation of the distal parts of dorsum of both hands |
TKI: Tyrosine kinase inhibitors; GIST: gastrointestinal stromal tumor; CD: cluster of differentiation
In summary, hair depigmentation is an unusual side effect of pazopanib and other tyrosine kinase inhibitors. While the exact mechanisms are not fully understood, inhibition of melanogenesis and disruption of signaling pathways may play a role. Hair depigmentation may have implications for therapy success, but more research is needed. Managing this side effect involves providing support and counseling to patients, and future studies should explore the molecular mechanisms and potential biomarkers related to hair depigmentation.
Limitations of current knowledge
Although hair depigmentation is a well-documented side effect of pazopanib therapy, the current understanding of its underlying mechanisms and implications for therapy success remains limited. Further, research is needed to elucidate the precise causes of this phenomenon and to determine whether it can serve as a reliable predictor of treatment efficacy.
Future directions
Given the potential implications of hair depigmentation for therapy success, future research should focus on elucidating the molecular mechanisms underlying this side effect and identifying potential biomarkers that could predict the efficacy of pazopanib and other tyrosine kinase inhibitors. Such findings could have significant clinical implications, enabling the development of personalized treatment strategies for patients with metastatic renal cell carcinoma and other malignancies.
CONCLUSION
Hair depigmentation is a common yet intriguing side effect of pazopanib therapy in patients with metastatic renal cell carcinoma. Although the exact causes of this phenomenon remain unclear, it is likely that a combination of multiple mechanisms, including the inhibition of melanogenesis and disruption of the SCF/c-Kit signaling pathway, contributes to its occurrence. Despite its potentially distressing nature, hair depigmentation could serve as a valuable predictor of therapy success, warranting further investigation in future research.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil
References
- A comprehensive review of mammalian pigmentation: Paving the way for innovative hair colour-changing cosmetics. Biology. 2023;12:290.
- [CrossRef] [PubMed] [Google Scholar]
- A novel KIT mutation results in piebaldism with progressive depigmentation. J Am Acad Dermatol. 2001;44:288-92.
- [CrossRef] [PubMed] [Google Scholar]
- Hypopigmentation in an African patient treated with imatinib mesylate: A case report. J Natl Med Assoc. 2003;95:722-4.
- [Google Scholar]
- Gray hair associated with the multitargeted receptor tyrosine kinase inhibitor Pazopanib. Ann Dermatol. 2015;27:791.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid hair depigmentation in patient treated with pazopanib. BMJ Case Rep. 2018;2018:bcr2018224209.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid hair depigmentation following treatment with pazopanib for metastatic renal cell carcinoma: Case report. World J Innov Res. 2021;10:20-1.
- [Google Scholar]






