Translate this page into:
Pembrolizumab-induced resolving papulosquamous eruption: An enigmatic presentation in a patient with renal cell carcinoma
*Corresponding author: Avik Mondal, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, Delhi, India. avik.mondal11@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mondal A. Pembrolizumab-induced resolving papulosquamous eruption: An enigmatic presentation in a patient with renal cell carcinoma. Indian J Skin Allergy. 2024;3:119-21. doi: 10.25259/IJSA_3_2024
Abstract
Lenvatinib, a multiple tyrosine receptor kinase inhibitor, and pembrolizumab, an anti-programmed cell death protein 1 humanized antibody, both are indicated in the treatment of renal cell carcinoma (RCC). Both these agents rarely produce cutaneous adverse events. In this instance, a RCC patient who received both medications and experienced a papulosquamous eruption as an uncommon cutaneous side effect of both medications is being reported. The potential drug triggers preceded the symptom onset and the observed symptom resolved on discontinuation of pembrolizumab.
Keywords
Papulosquamous eruption
Pembrolizumab
Lenvatinib
Drug-related eruption
INTRODUCTION
The US Food and Drug Administration approved the oral multiple tyrosine receptor kinase inhibitor lenvatinib for the treatment of thyroid cancer, hepatocellular carcinoma (HCC), endometrial carcinoma, and renal cell carcinoma (RCC).[1] Immune checkpoint inhibitors are highly selective drugs that take advantage of critical biochemical pathways that cancerous cells use to sidestep the host immune response. The first anti-programmed cell death protein 1 (PD-1) humanized antibody authorized for use in cancer treatment, pembrolizumab, functions by blocking the PD-1/PD-1L axis, beneficial in the treatment of melanoma, non-small-cell lung cancer and also in RCC.[2] Here, a case of RCC receiving both agents developed papulosquamous eruption, which is a rare cutaneous adverse event of both agents. Withdrawal of pembrolizumab resulted in spontaneous resolution of the lesions, and the patient was continued on lenvatinib.
CASE REPORT
A 61-year-old male patient of intermediate risk RCC presented with generalized, erythematous, moderately itchy, scaly plaques and papules (Common terminology criteria for adverse event, CTCAE grade 2) after receiving an injection of pembrolizumab 200 mg. Simultaneously, he was also receiving lenvatinib 20 mg once daily orally, which was initiated 3 days before receiving pembrolizumab. The eruption started from the upper and lower limbs and subsequently involved the trunk. There was no history of frank pustulation or of burning sensation. On detailed history, it was found that he had received both agents 2 months back, and at that time, he experienced a similar eruption in lesser severity that developed 7–10 days after treatment initiation. In the previous episode, both medications were stopped in view of skin eruption (Naranjo score 4) in the oncology department, which led to subsidence without any specific treatment. In between the two episodes, he did not develop any cutaneous lesions. He did not give a history of prior upper respiratory tract infection, simultaneous mucosal ulceration, facial edema, or scalp involvement in both episodes.
On examination, generalized involvement of the body sparing the face and scalp in the form of erythematous, scaly, well to ill-defined, papules and plaques along with peripheral white, semi-adherent scaling resembling collarette scales was observed [Figures 1 and 2]. Some lesions were arcuate to annular and maximum involvement over the dorsum of the feet was evident. Over the trunk, lesions were papular, ill-defined, less erythematous, and scaly than the limbs.
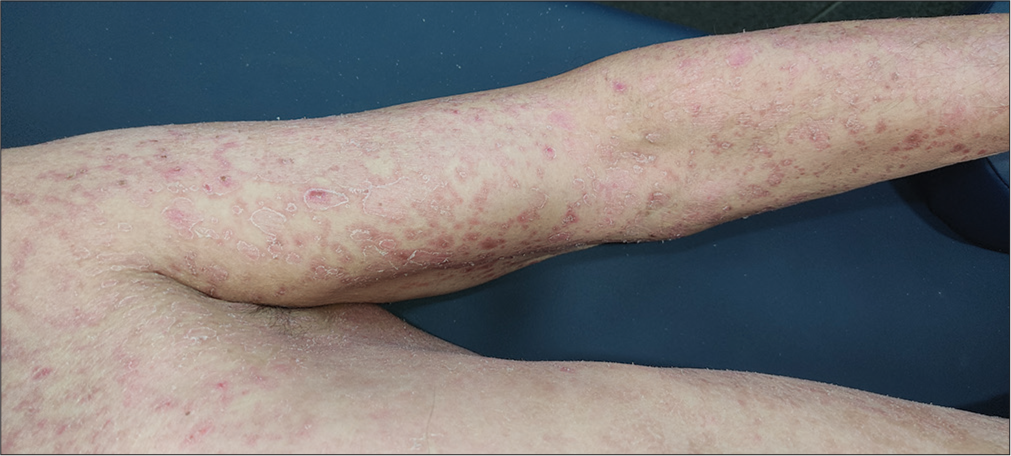
- Multiple well to ill-defined erythematous annular plaque and papules with peripheral whitish scaling over the chest, left arm, and forearm.
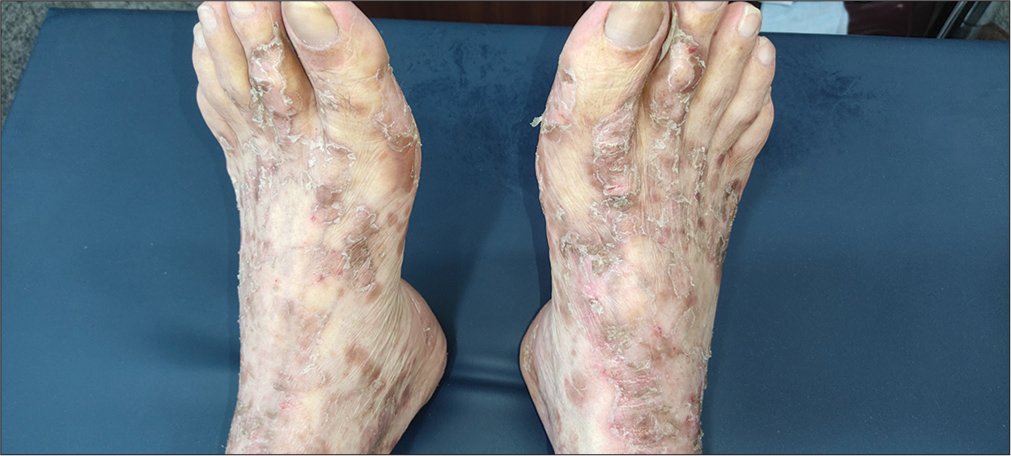
- Erythematous whitish scaly, well-defined linearly arranged plaque over the dorsum of feet bilaterally.
All the blood investigations, including peripheral smears, were within normal limits. The patient refused to undergo a skin biopsy. Pembrolizumab was stopped, and the patient was continued on lenvatinib 20 mg. He was advised to apply emollients liberally and prescribed a twice-daily oral antihistamine (levocetirizine 5 mg) to alleviate itching. The patient presented after 4 weeks with significant improvement in terms of resolving erythematous, papules and plaques and pruritus [Figures 3 and 4] and did not have any further episodes on follow-up.
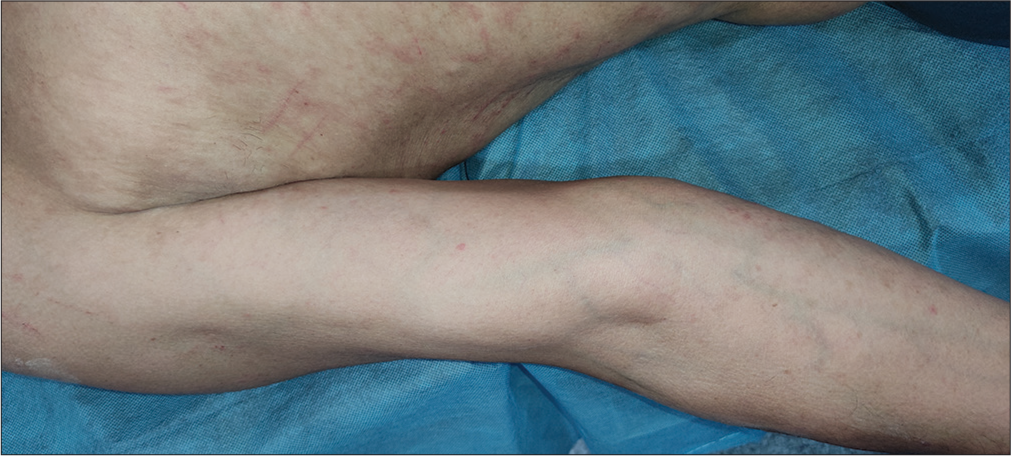
- Almost resolved skin eruption after 4 weeks of stopping pembrolizumab.
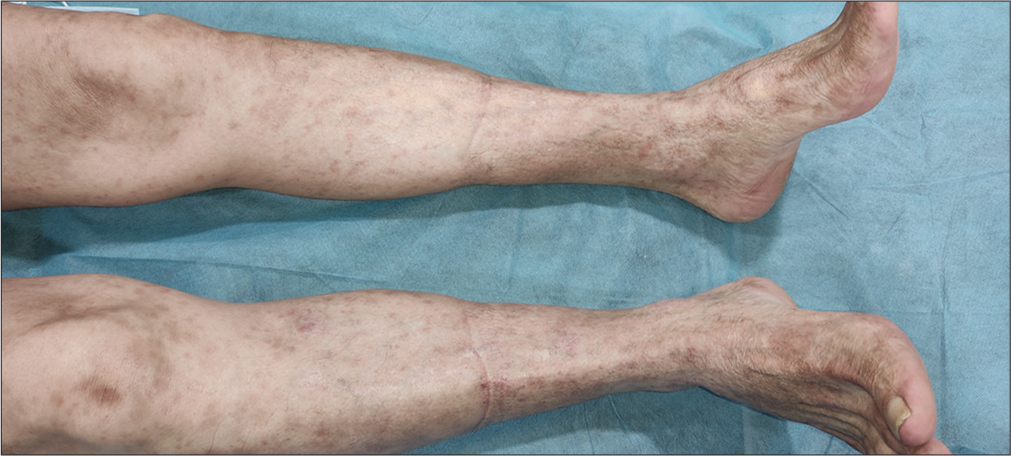
- Resolved skin lesion over lower limbs with residual post-inflammatory hyperpigmentation.
DISCUSSION
Although anti-PD-1 medications are usually well tolerated, between 18% and 42% of patients experience cutaneous adverse effects. The most common cutaneous adverse effects among patients receiving pembrolizumab are reported to be maculopapular eruption, pruritus, and hypopigmentation or vitiligo-like lesions.[3,4] Common dermatological adverse events caused by lenvatinib include xerosis, hair and skin discoloration, and palmoplantar dysesthesia.[5] Lenvatinib-induced psoriasiform eruption and palmoplantar erythema in a patient of HCC were first reported by Sally et al.[6] in 2021. A recent report described two patients with psoriasiform rash attributed to nivolumab for stage IV melanoma and to pembrolizumab and TAK-981 for stage IV colorectal cancer. Remarkably, the biopsy findings were an overlap of psoriasiform, spongiotic, and lichenoid dermatitis. The rash cleared partially with apremilast in the first patient, and in the second patient, ixekizumab resulted in a complete resolution. In the present case, a biopsy was not carried out; however, the temporal correlation of the onset of rash after pembrolizumab and the complete resolution after its withdrawal indicates its causality. The patient could be continued on lenvatinib uneventfully.
CONCLUSION
Understanding immune-mediated cutaneous adverse effects in cancer patients would facilitate early detection and allow us to either change the regimen or decrease the dose, thereby improving the quality of life. The present case report describes not only a rare cutaneous adverse event due to anti-PD-1 but also shows the importance of detailed history elicitation and stoppage of the suspected drug that would facilitate the complete resolution of lesions.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Lenvatinib in management of solid tumors. Oncologist. 2020;25:e302-10.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting the PD1/PD-L1 axis in melanoma: Biological rationale, clinical challenges and opportunities. Crit Rev Oncol Hematol. 2014;89:140-65.
- [CrossRef] [PubMed] [Google Scholar]
- Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-12.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: A dermatology perspective on management [Formula: See text] J Cutan Med Surg. 2021;25:59-76.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous side effects of molecularly targeted therapies for the treatment of solid tumors. Drugs Context. 2018;7:212516.
- [CrossRef] [PubMed] [Google Scholar]
- Lenvatinib-induced psoriasiform eruption and palmoplantar erythema in a patient with hepatocellular carcinoma. JAAD Case Rep. 2021;15:1-3.
- [CrossRef] [PubMed] [Google Scholar]






