Translate this page into:
Pacemaker dermatitis – A rare hypersensitivity reaction
*Corresponding author: Arunima Ray, Department of Dermatology, Narayana Health - Rabindranath Tagore Institute of Cardiac Sciences, Kolkata, West Bengal, India. arunima.roma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ray A, Sengupta C. Pacemaker dermatitis – A rare hypersensitivity reaction. Indian J Skin Allergy. 2025;4:87-8. doi: 10.25259/IJSA_55_2024
A 68-year-old male presented with a well-defined area of circumscribed, eczematous plaque [Figure 1] over the left side of the chest. He reported that 12 weeks before the development of the rash, he had a cardiac pacemaker installation for a 2:1 atrioventricular block with intermittent complete heart block. It was a dual chamber rate modulated pacemaker (3.0T) composed of titanium, chromium, and nickel. There was no history of any topical applications, dye usage, perfumes, or sprays before the appearance of the rash. The lesion was restricted to the area of the underlying device. Differentials considered included dry discoid eczema and papulosquamous disorders including psoriasis. Dermoscopy [Figure 2] was done and demonstrated fine whitish scales, irregular red dots, and peripheral scales against a brown blotchy background. Based on the morphology, history of pacemaker installation, and dermoscopic features of eczema – including red dots and scales consonant with dilated capillaries in elongated dermal papillae, and hyperkeratosis, respectively, a diagnosis of eczema due to pacemaker was made.[1] A diagnostic biopsy and patch test were advised; however, the patient firmly declined any further investigations despite being counseled about the need for these investigations and the potential consequences of not performing the same. The patient was prescribed medium potency topical steroids and emollients, with oral levocetirizine 5 mg for 2 weeks, and was advised to follow-up at 2 weeks. He was unable to follow up physically but continued to report improvement telephonically over 6 months, while the pacemaker remained in-situ.
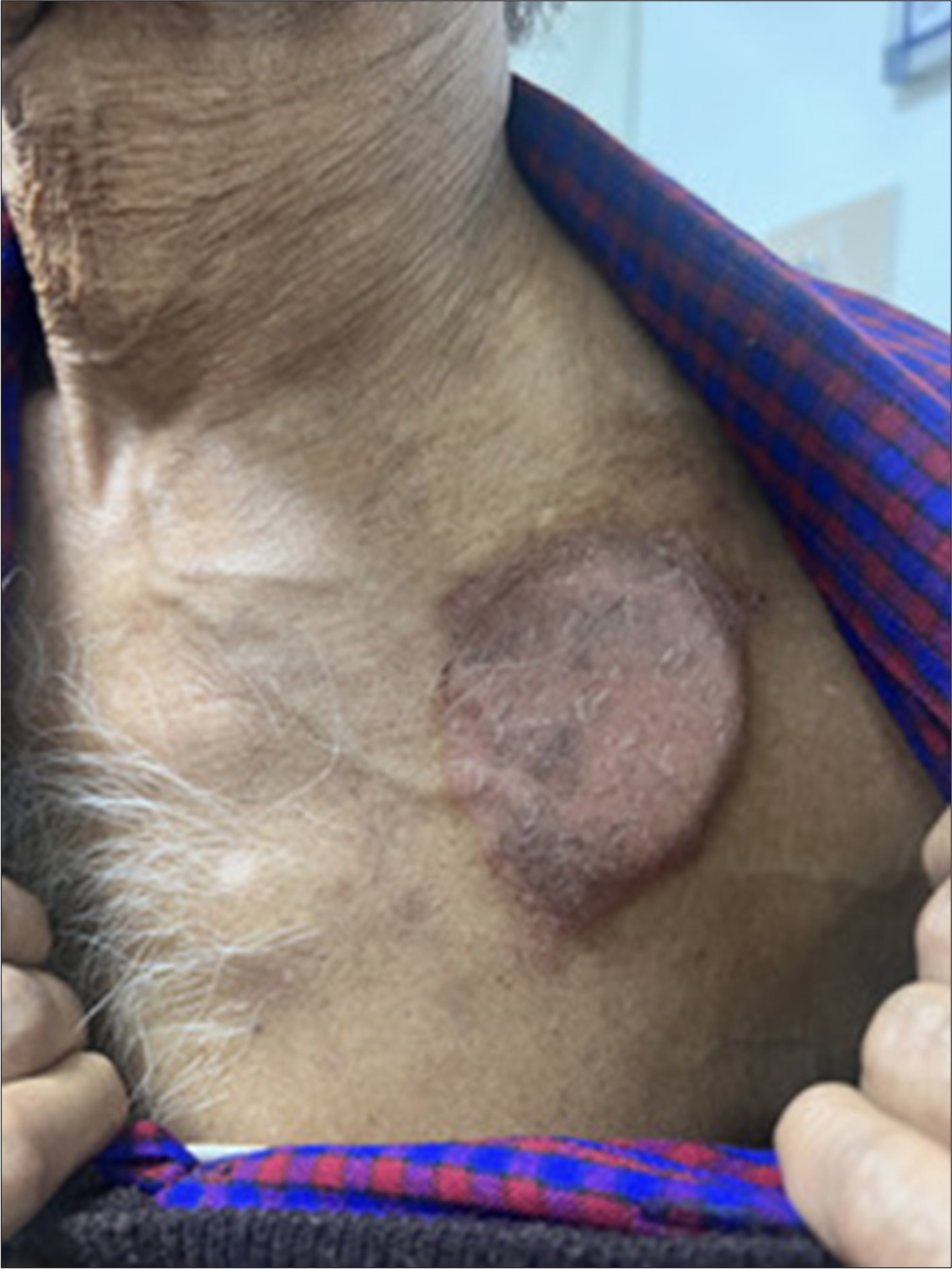
- Circumscribed area of dermatitis overlying the area where the cardiac pacemaker – (Dual chamber rate modulated – 3.0T) composed of titanium, chromium, and nickel – was installed.
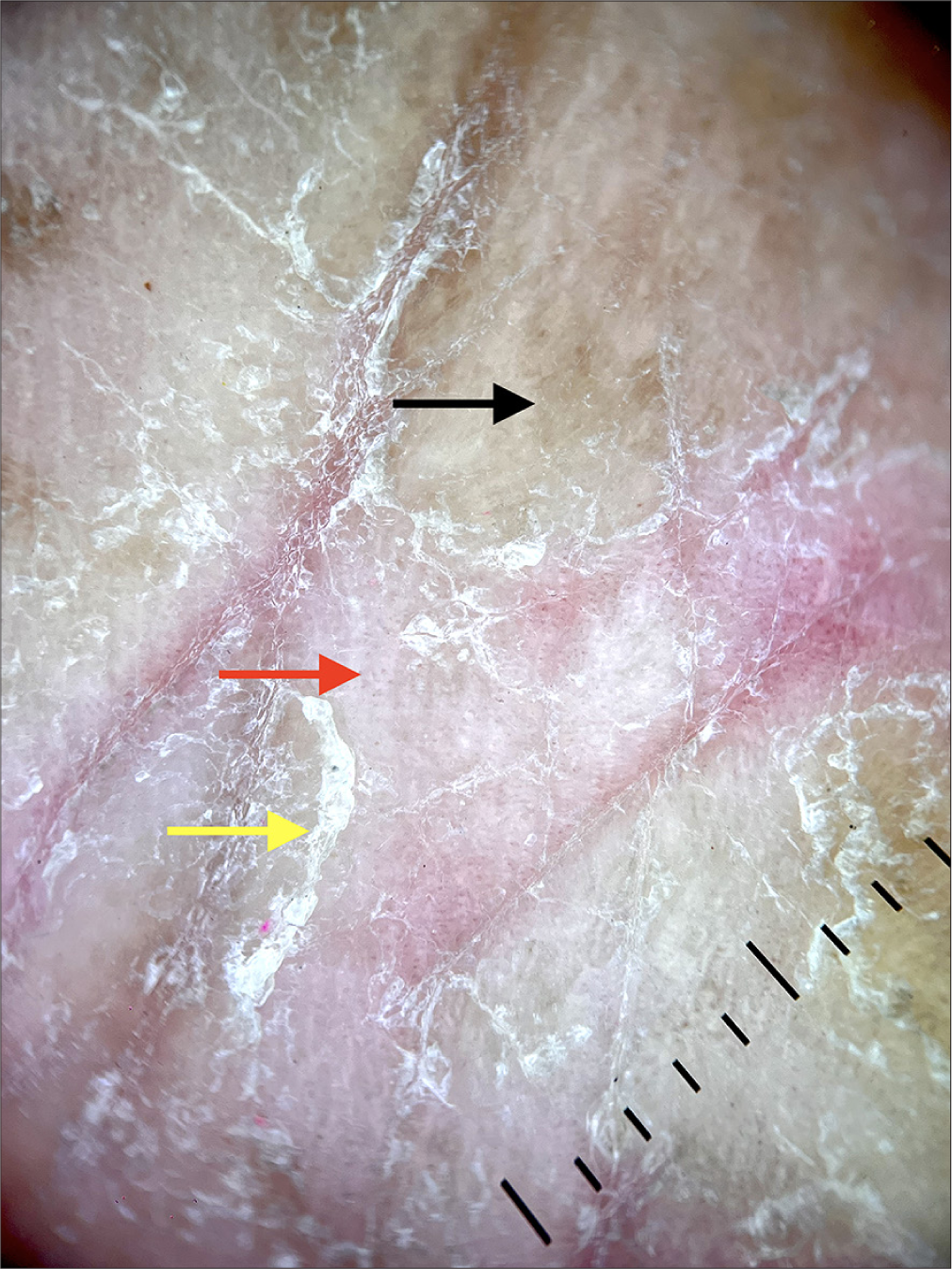
- Dermoscopy (DermLite DL4, ×100, polarized light) shows irregular red dots (red arrow), and whitish scales (yellow arrow), with fine peripherally attached scales with a brownish background (black arrow).
Although rarely seen, limited cases of pacemaker dermatitis have been described as was reported by Dogan et al. Further, they noted that topical steroids were effective, and no relapse was seen.[2] Ljubojević Hadžavdić et al. reported a case of localized pacemaker dermatitis, which was morphologically similar to local tissue infection, suggesting a need to rule out infections with a tissue swab culture. The components of a pacemaker include a generator and the leads with composite elements of titanium, nickel, polyurethane, epoxy, mercury, cadmium, chromium, silicon, and cobalt. Of these, nickel is the most frequently culpable.[3] Previously reported cases have shown full resolution with the removal of the metal implants. Pacemaker hypersensitivity may show severe systemic features as was seen in an 80-year-old male with erythroderma, reported by Stringer et al. The reported patient was suspected to have hypersensitivity to titanium, silicone, or other internal components of the device. The authors proposed that the management should include the removal of the system in case of a severe reaction and substitution with alternate hypoallergenic gold-coated pacemakers or an epicardial system or use of a polytetrafluoroethylene sheet to wrap generators and leads.[4] A greater awareness could prevent greater morbidity and obviate the need for more invasive investigations and unnecessary treatment. In addition, in patients with a prior history of allergies, a well-timed patch test may be required before invasive implantations, especially in patients with an atopic background.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Dermoscopy of inflammatory dermatoses (inflammoscopy): An up-to-date overview. Dermatol Pract Concept. 2019;9:169-80.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis after implantable cardiac defibrillator implantation for ventricular tachycardia. Intractable Rare Dis Res. 2016;5:56-7.
- [CrossRef] [PubMed] [Google Scholar]
- Severe erythroderma secondary to permanent pacemaker allergy. HeartRhythm Case Rep. 2021;7:207-10.
- [CrossRef] [PubMed] [Google Scholar]






