Translate this page into:
Comparative assessment of quality of life in chronic spontaneous urticaria receiving second generation anti histamines: A real world study
*Corresponding author: Dhiraj Dhoot, Global Medical Affairs, Glenmark Pharmaceuticals Limited, Mumbai, Maharashtra, India. dhiraj.dhoot@glenmarkpharma.com
-
Received: ,
Accepted: ,
How to cite this article: Shah B, Choudhary A, Jangid N, Mistry D, Shah S, Kamat S, et al. Comparative assessment of quality of life in chronic spontaneous urticaria receiving second generation anti histamines: A real world study. Indian J Skin Allergy 2022;1:7-10.
Abstract
Objectives:
Chronic spontaneous urticaria (CSU) is correlated with a high detrimental effect on the quality of life (QoL). Antihistamines are the first choice drugs in the management of CSU. QoL is important in the evaluation of the efficacy of antihistamines, as these are the most commonly used in CSU.
Materials and Methods:
In this comparative, three-arm study, patients with CSU were randomized to standard dose of either bilastine, fexofenadine, or levocetirizine for a period of 4 weeks. Patients were assessed for improvement in their QoL based on chronic urticaria QoL questionnaire (CU-Q2oL) questionnaire and urticaria activity score (UAS).
Results:
Fifty-eight CSU patients were randomized to bilastine (n = 23), fexofenadine (n = 18) and levocetrizine (n = 17) groups. There was significant improvement in CU-Q2oL and UAS score in all the groups during study period. 83%, 72%, and 65% patients reported improvement in CU-Q2oL score in bilastine, fexofenadine, and levocetrizine group, respectively. Bilastine was associated with significant improvement in CU-Q2oL compared to fexofenadine and levocetrizine (P < 0.05). Mean reduction in UAS score was 86%, 77%, and 68% in bilastine, fexofenadine and levocetrizine group respectively. The difference was statistically insignificant between the groups. The CU-Q2oL total score correlated more strongly (r = 0.62; P = 0.001) with the UAS7 in bilastine group than fexofenadine (r = 0.57; P = 0.01) and levocetrizine groups (r = 0.53; P = 0.02).
Conclusion:
The results of the study proved that, in CSU patients, QoL was improved significantly with bilastine as compared to fexofenadine and levocetirizine.
Keywords
Urticaria
Quality of life
Bilastine
Levocetrizine
Fexofenadine
Real world
INTRODUCTION
Chronic spontaneous urticaria (CSU), being a chronic disease with fluctuating severity of the symptoms requires stern criteria to determine the disease activity and disease-specific deterioration on the day to day activity in terms of quality of life (QoL), and the association of these factors with treatment.[1] For CSU, two types of assessments are already in place; first, clinical scoring tool and second, QoL questionnaires. Among all available clinical scoring tools, the most effective is the Urticaria Activity Score (UAS7), based on the daily appraisal of urticarial symptoms over a week.[2] Since considerable variations are seen in urticaria symptoms from day to day; to ensure results of UAS, it becomes prudent to document symptoms activity for several days in a row.
QoL assesses the functional and physical status of a disease and relative treatment from the specific aspect of the patient. Therefore, QoL is a subjective concept and has become an important tool in assessing the comparative efficacy of multiple treatment options in all chronic diseases.[3] QoL is especially important in CSU since CSU is associated with low mortality but a high pernicious effect on the QoL including daily activities such as mobility, home and work management, and emotional well-being.[4-6] Thus, QoL forms important part in the clinical assessment of these patients, and it is widely used in numerous clinical trials and real-world evidence studies.
QoL is important in the evaluation of the efficacy of antihistamines, as these are the most commonly used in CSU. In multiple studies, the correlation between the clinical or symptom scores and QoL questionnaire scores has been found to be moderate in CSU (r = 0.64–0.69).[7] This asserts the discrepancy between the symptom severity and subjective impression of the impact of these symptoms in real-world life. Nevertheless, in few comparative studies, it has been found that CSU patients are more affected than patients with other allergic disorders in their daily life.[8]
This study compared the efficacy of bilastine, fexofenadine, and levocetirizine in CSU at standard dose and their impact on QoL. We correlated the Chronic Urticaria QoL Questionnaire (CU-Q2oL)[9] with UAS7 in assessing the clinical response of these drugs in CSU.
MATERIALS AND METHODS
The present study was conducted at the tertiary care hospital in Ahmedabad from April 2020 to October 2020 where 58 patients comprising 26 females and 32 males with age group range of 18–65 years. Only previously diagnosed CSU patients with UAS7 score of a minimum of 7 in the preceding week were enrolled in the study. All the patients were randomly divided into three groups. Group I received bilastine 20 mg/day, Group II received fexofenadine 180 mg/day whereas Group III received levocetirizine 5 mg/day for 4 weeks. After the clinical examination on day 1, all the patients completed CU-Q2oL questionnaire to estimate the effect of CSU on their day-today life and were randomized into three groups to receive respective treatment. After completion of treatment of 4 weeks, all the patients again completed their second self-assessment with CU-Q2oL. Patients were evaluated as;
Remarkable improvement - UAS7 = 0 and >25% improvement in CU-Q2oL
Improvement – UAS7 <6 and <25% improvement in CU-Q2oL
No change or worsening of symptoms – UAS7 >6 and No improvement in baseline CU-Q2oL total score.
Results were presented as mean scores, and groups were compared using One Way ANOVA with Tukey HSD test. Correlations were calculated using the Pearson correlation test. The study was approved by the institutional ethics committee and was registered on the clinical trials registry, India (CTRI/2020/03/024244).
RESULTS
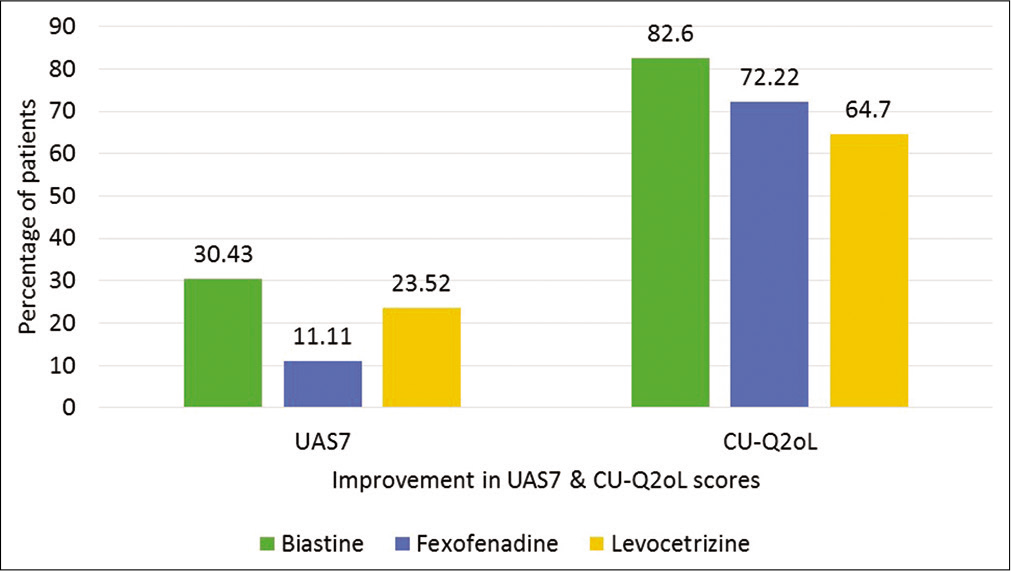
Out of 58 patients, 23 were enrolled in Group I, 18 in Group II, and 17 in Group III. The mean scores for clinical scoring tool (UAS7) and QoL questionnaire (total CUQ2oL score and CU-Q2oL domain score) before and after treatment are cited in Tables 1 and 2, respectively. The percentage of patients showing remarkable improvement in their UAS7 and CU-Q2oL score, is shown in Figure 1.
| Assessment | Drugs | ||
|---|---|---|---|
| Bilastine | Fexofenadine | Levocetrizine | |
| N | 23 | 18 | 17 |
| M | 13 | 9 | 10 |
| F | 10 | 9 | 7 |
| Age | 33.34±11.64 | 34.77±11.34 | 36.52±11.57 |
| UAS7 | 17.27±3.60 | 19.41±3.64 | 19.44±4.03 |
| CU-Q2oL | |||
| Total | 49.38±11.40 | 49.88±10.87 | 47.38±11.34 |
| Pruritus | 6.96±1.84 | 7.41±1.33 | 7.06±1.48 |
| Swelling | 3.04±1.78 | 3.59±1.91 | 3.56±2.25 |
| Impact on life activities | 13.54±4.15 | 13.24±3.87 | 12.0±4.43 |
| Sleep problems | 10.31±3.07 | 11.06±3.03 | 10.0±3.63 |
| Limits | 6.23±1.86 | 5.65±1.66 | 5.50±1.83 |
| Looks | 9.31±2.41 | 8.94±2.79 | 9.25±2.96 |
UAS: Urticaria activity score, CU-Q2oL: Chronic urticaria quality of life questionnaire
| Assessment | Drugs | ||
|---|---|---|---|
| Bilastine | Fexofenadine | Levocetrizine | |
| UAS7 | 2.40±2.10 | 4.53±3.39 | 6.31±2.98 |
| CU-Q2oL | |||
| Total | 28.65±10.62 | 37.47±6.65 | 34.81±7.39 |
| Pruritus | 3.96±1.27 | 4.71±0.99 | 4.63±1.20 |
| Swelling | 2.38±0.71 | 2.59±1.00 | 2.75±1.44 |
| Impact on life activities | 8.33±2.55 | 10.53±2.43 | 9.0±2.50 |
| Sleep problems | 6.50±1.74 | 8.71±2.28 | 6.81±1.80 |
| Limits | 3.92±0.97 | 4.53±1.12 | 4.63±1.41 |
| Looks | 5.96±1.20 | 6.41±1.37 | 7.0±2.22 |
UAS: Urticaria activity score, CU-Q2oL: Chronic urticaria quality of life questionnaire
- Improvement in individual scores.
The primary objective was to compare UAS7 and CU-Q2oL assessments in all groups. The baseline mean UAS7 score in all groups was below 20 suggesting poor or ineffective response with previous therapy. But on adjusting the therapy, there was remarkable response in all the groups. In Group I, 86% improvement in the mean UAS7 score was noted with 30% of patients showing clearance of the symptoms, while in Group II, there was 77% improvement in mean UAS7 score with 11% of the patients with complete clearance of symptoms. Similar trend was seen in Group III with 68% improvement in mean UAS7 score and 23% of the patient with complete clearance of symptoms. All the results were statistically significant (P < 0.05). But on comparison with groups, there was no statistical difference in any of the treatment arm.
Similarly, following treatment adjustment, 83% of the patients reported improvement in CU-Q2oL score in Group I, 72% in Group II, and 65% in Group III. Significant change in CUQ2oL total score was seen albeit 25% improvement criteria from baseline suggesting significant clinical improvement with a change of therapy. On inter-group comparison, there was statistical difference between Group I and II and Group I and III (P < 0.05). There was no statistical difference between Group II and III (P= 0.17).
Furthermore, improvement was seen in all domains of CUQ2oL in all groups [Table 3]. In Group I, strong correlation of CU-Q2oL total score was noted with UAS7 (r = 0.62; P= 0.001) than Group II (r = 0.57; P= 0.01) and Group III (r = 0.53; P= 0.02). Moreover, on further analysis, significant correlations was seen with UAS7 for all domains of CUQ2oL in Group I, sleep problems and limits in Group II and pruritus, sleep problems, looks, and limits in Group III as shown in Table 3.
| CU-Q2oL domains | Bilastine | Fexofenadine | Levocetrizine | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Pruritus | 0.4 | 0.06 | 0.12 | 0.63 | 0.55 | 0.02 |
| Swelling | 0.4 | 0.06 | 0.07 | 0.75 | 0.12 | 0.62 |
| Impact on life activities | 0.42 | 0.04 | 0.07 | 0.77 | 0.12 | 0.63 |
| Sleep problems | 0.45 | 0.03 | 0.29 | 0.23 | 0.34 | 0.17 |
| Limits | 0.52 | 0.01 | 0.42 | 0.07 | 0.71 | 0.001 |
| Looks | 0.48 | 0.02 | 0.16 | 0.51 | 0.47 | 0.055 |
CU-Q2oL: Chronic urticaria quality of life questionnaire
DISCUSSION
The guidelines for urticaria published by EAACI/GA2LEN/ EDF/WAO and Japanese Dermatological Association accentuate the importance of QoL for preferred added therapy in refractory cases.[10,11] Comparisons between patients with CSU and other allergic diseases highlighted that CSU patients were more affected than patients with other allergic diseases in their daily life, in relation to sleep, occupational behavior, and general physical and psychological functioning.[8]
Different other QoL questionnaires have been studied in CSU, but the CU-Q2oL is the only questionnaire that assesses CSU-specific QoL.[12] It contains 23 questions in 6 different domains (pruritus, swelling, impact on life activities, sleep problems, limits, and looks). In general, each question or statement is answered on 5 point scale (1 = not at all; 5 = very much); the score is calculated adding the score for each statement or question, with a minimum score of 23 and a maximum score of 115, higher scores imply poorer QoL.
In one study,[13] the effectiveness of bilastine in relation to QoL in CSU had been compared with levocetrizine. In that study, QoL was assessed with Dermatology Life Quotient Index (DLQI) score, and improvement was observed in total DLQI score as well as individual domains of DLQI score (P < 0.001), with no statistical difference between both the drugs. But in our study, bilastine was found to be statistically significant than both the drugs in the improvement of QoL.
Apart from histamine, there are many other mediators which play important role in manifestations of CSU. Hence it is possible that the anti-inflammatory properties of bilastine may be responsible for significantly vitiating the CSU symptoms leading to improvement in QoL. Although, there is no data on anti-inflammatory properties of bilastine in humans, prelusive findings from in-vitro studies demonstrated the anti-inflammatory effects of bilastine.[13] Moreover, animal studies have exhibited the anti-allergic effects of bilastine similar to cetirizine and fexofenadine.[14]
The only limitation in this study is the relatively small sample. However, this does not affect the results of the study. From this study, it can be concluded that in the management of CSU, CU-Q2oL is an important tool to identify the changes in QoL related with disease activity. Since QoL is of utmost importance, we would like to recommend that both; clinical scoring tool (UAS7) and QoL questionnaire (CU-Q2oL) should be used frequently for clinical assessment in CSU.
CONCLUSION
From this study, it can be concluded that CU-Q2oL is a valid tool to detect changes in QoL associated with CSU. Additionally in this study, quality of life was improved significantly with bilastine as compared to fexofenadine and levocetrizine. Since patient QoL is of eminent importance, we recommend to use the UAS7 and CU-Q2oL scoring tool regularly for assessing patient responses to therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Dhiraj Dhoot, Dr. Gaurav Deshmukh and Dr. Hanmant Barkate are employees of Glenmark Pharmaceuticals Ltd.
References
- Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317-30.
- [CrossRef] [PubMed] [Google Scholar]
- How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80.
- [CrossRef] [PubMed] [Google Scholar]
- Bilastine and quality of life. J Investig Allergol Clin Immunol. 2011;21(Suppl 3):16-23.
- [Google Scholar]
- Burden of chronic urticaria relative to psoriasis in five European countries. J Eur Acad Dermatol Venereol. 2018;32:282-90.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of sedative and non-sedative antihistamines on the impaired productivity and quality of life in patients with pruritic skin diseases. Allergol Int. 2010;59:345-54.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol. 2005;152:289-95.
- [CrossRef] [PubMed] [Google Scholar]
- The urticaria severity score: A sensitive questionnaire/index for monitoring response to therapy in patients with chronic urticaria. Ann Allergy Asthma Immunol. 2009;102:475-82.
- [CrossRef] [Google Scholar]
- Quality of life and patients' satisfaction in chronic urticaria and respiratory allergy. Allergy. 2003;58:621-3.
- [CrossRef] [PubMed] [Google Scholar]
- A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL) Allergy. 2005;60:1073-8.
- [CrossRef] [PubMed] [Google Scholar]
- The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
- [CrossRef] [PubMed] [Google Scholar]
- Japanese guidelines for diagnosis and treatment of urticaria in comparison with other countries. Allergol Int. 2012;61:517-27.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic spontaneous urticaria: how to assess quality of life in patients receiving treatment. Arch Dermatol. 2011;147:1221-3.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: A multi-centre, double-blind, randomized, placebo-controlled study. Allergy. 2010;65:516-28.
- [CrossRef] [PubMed] [Google Scholar]
- Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: Receptor selectivity and in vitro antihistaminic activity. Drugs R D. 2005;6:371-84.
- [CrossRef] [PubMed] [Google Scholar]






