Translate this page into:
Phototoxicity and photoallergy – A brief review on pathogenesis, recent advances, and approach to a patient with photosensitivity
*Corresponding author: Vinayak N. Anchan, Assistant Professor, Department of Dermatology, Venereology and Leprosy, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India. vinayakanchan1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pai SB, Anchan VN. Phototoxicity and photoallergy – A brief review on pathogenesis, recent advances, and approach to a patient with photosensitivity. Indian J Skin Allergy. 2024;3:28-35. doi: 10.25259/IJSA_9_2024
Abstract
Photosensitivity can result in phototoxic or photoallergic reactions. Phototoxicity is the more frequent of the two and could be acute or chronic. Clinically, erythema and edema are the hallmarks of acute phototoxic reactions. There are two mechanisms by which phototoxic reactions can happen; photodynamic (which is oxygen dependent) and non-photodynamic (which is oxygen independent). Ultraviolet B (UVB) and less effectively UVA can cause phototoxic reactions. The main action spectrum for chronic phototoxicity falls in the UVB range, as compared to the UVA rays. Other phototoxic reactions include lichenoid or lichen planus such as reaction and photo-onycholysis. Photoallergic reactions usually are due to delayed hypersensitivity reaction that manifests with papular or eczematous eruptions. Topical medications are typically the most frequent provoking cause of photoallergic skin lesions. A thorough history with detailed clinical examination is necessary for the management of photosensitivity. Availability of newer diagnostic techniques and treatment modalities gives a promising approach to the management of photo-aggravated dermatoses.
Keywords
Phototoxicity
Photoallergy
Photosensitivity
Photodermatoses
INTRODUCTION
Photosensitivity refers to a hyperbolic reaction to ultraviolet (UV) light that produces symptoms such as burning, itching, and redness. Various topical or systemic exogenous agents can cause photosensitivity. There are many potential complications and lacunae associated with photosensitivity, making it a difficult area of dermatology for both patients and doctors. This review will briefly discuss the effects of light on the skin.
Light-skin interactions
Light has both the properties of waves and particles known as photons. They can undergo reflection, scattering, or absorption. Energy is transferred from radiation-absorbing molecules in the skin, known as chromophores, to drive photochemical reactions or produce heat. This procedure produces observable cellular and molecular reactions that may result in a therapeutic effect. The light must be of the appropriate wavelength to be absorbed by the target chromophore to exert a clinical effect.
Photochemical reactions
When the chromophore absorbs the photon, it changes to a transient, excited state. Energy is released as light or heat when the chromophore returns from the excited state to the ground state. Only absorbed light can lead to a photochemical reaction, causing cellular changes, which eventually evoke a clinical response.
Acute and chronic effects of ultraviolet radiation (UVR)
After UVR exposure, sunburn cells, or apoptotic keratinocytes, can be observed histologically. Sunburn cells can be seen as early as half an hour after UVR. This is a protective mechanism of the body to get rid of cells that may be at risk for malignant transformation. Compared to melanocytes, keratinocytes are more susceptible to UVR. This is due to the fact that melanocytes undergo fewer cycles than keratinocytes. During the process of synthesizing DNA, cells are more susceptible to apoptosis.
Over several years, extensive changes such as wrinkles, atrophy, hyper- and hypopigmented macules, telangiectasia, yellow papules and plaques, keratotic growths (actinic keratoses), and occasionally the development of skin cancer are caused by repeated exposure to the sun, especially ultraviolet A (UVA) rays. Histologically, there may be numerous aberrant keratinocytes arranged in an uneven manner, effacement as well as projections of the rete ridges, and a potential thinning of the epidermis.
Photosensitivity can be phototoxic or photoallergic. Phototoxicity is the more frequent of the two. Negative sun-induced skin reactions are becoming more common as a result of the numerous photosensitizing chemicals that are entering our environment from various sources. The term “photosensitivity” refers broadly to the negative effects.[1]
PHOTOTOXICITY
Acute phototoxicity
Phototoxicity is frequent, much like toxic reactions in general. If sufficient amount of the chromophore absorbs sufficient amount of the light radiation in reactive tissue, then phototoxic reactions could happen in all individuals.[2] Clinically, erythema and edema are the hallmarks of these reactions; they are unpleasant and typically manifest later. After exposure, the erythema often appears a few hours later, peaks in a few hours or days, and is then followed by hyperpigmentation and desquamation. “Sunburns” caused by ultraviolet B (UVB), which ranges from 290 nm to 320 nm, are typical cases of acute idiopathic phototoxic reactions caused, at least partially, by skin-based DNA molecules absorbing these photons.[3] Acute erythema has also been demonstrated to be caused by UVA radiation (340–400 nm); however, these rays are more melanogenic than erythrogenic. Chemical photosensitization, resulting from exposure to the appropriate action spectrum of rays, can cause early or immediate burning and stinging reactions in the skin. Examples of these compounds are coal tar, demethylchlortetracycline, and porphyrins [Table 1]. Phototoxic effects are enhanced by wind and humidity.[4] Owens and Knox, examined the effects of humidity, wind, and temperature on UVR-induced acute and chronic skin damage in test animals kept in environmental chambers and exposed to radiation under carefully regulated circumstances. They noticed accelerated skin damage in animals that were exposed to UVR, subsequent to the increase in these parameters. Moreover, because infrared light (760–3000 nm) represents a portion of the sun’s spectrum that reaches the earth’s surface, it can also cause acute toxic cutaneous changes including erythema and edema [Figure 1].
| Phototoxicity | Photoallergy | |
|---|---|---|
| Clinical features | Reaction akin to sunburn, erythema, edema with bullae and vesicles | Eczematous lesion with pruritus |
| Mechanism | Cellular damage by direct tissue injury | Delayed hypersensitivity reaction |
| Histopathology | Necrotic keratinocytes, dermal edema with neutrophilic, lymphocytic and macrophagic infiltrate | Spongiotic dermatitis, dermal lymphohistiocytic infiltrate |
| Medications | Chlordiazepoxide, Griseofulvin, Voriconazole, Ciprofloxacin, Tetracyclines, Furosemide, Thiazide, Naproxen, Ibuprofen | Benzophenone-3, 4 Padimate-O, A Homosalate, PABA, Avobenzone, 6 methylcoumarin, Musk ambrette, Sandalwood oil |
| MED A | Low (systemic) | Low (systemic) |
| MED B | Normal | Normal |
| Onset | Minutes to hours after exposure | Starts 24–48 h after exposure |
| Cross reactivity | None | Common |
| Diagnosis | Clinical and patch test | Clinical and photopatch test |
MED: Minimal erythema dose, PABA: Para-aminobenzoic acid
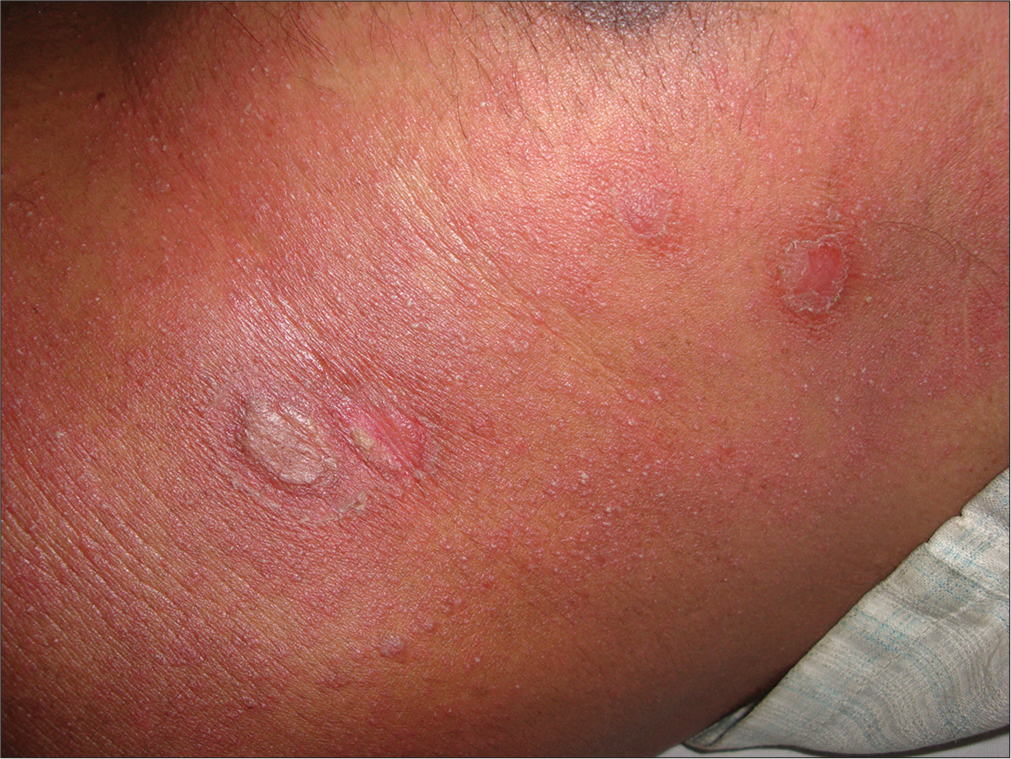
- Phototoxic reaction to 8-methoxypsoralen in a patient with psoriasis.
Mechanisms
Numerous photochemical reactions can result in interactions between non-ionizing radiation, photosensitizing chemicals, and the affected biological structures; however, the damage to these biological substrates is initiated by the photosensitizer’s absorption of the appropriate wavelengths (action spectrum) [Figure 2]. Generally speaking, there are two types of phototoxic reactions that can happen: photodynamic (that is oxygen dependent) and non-photodynamic (that is oxygen-independent).
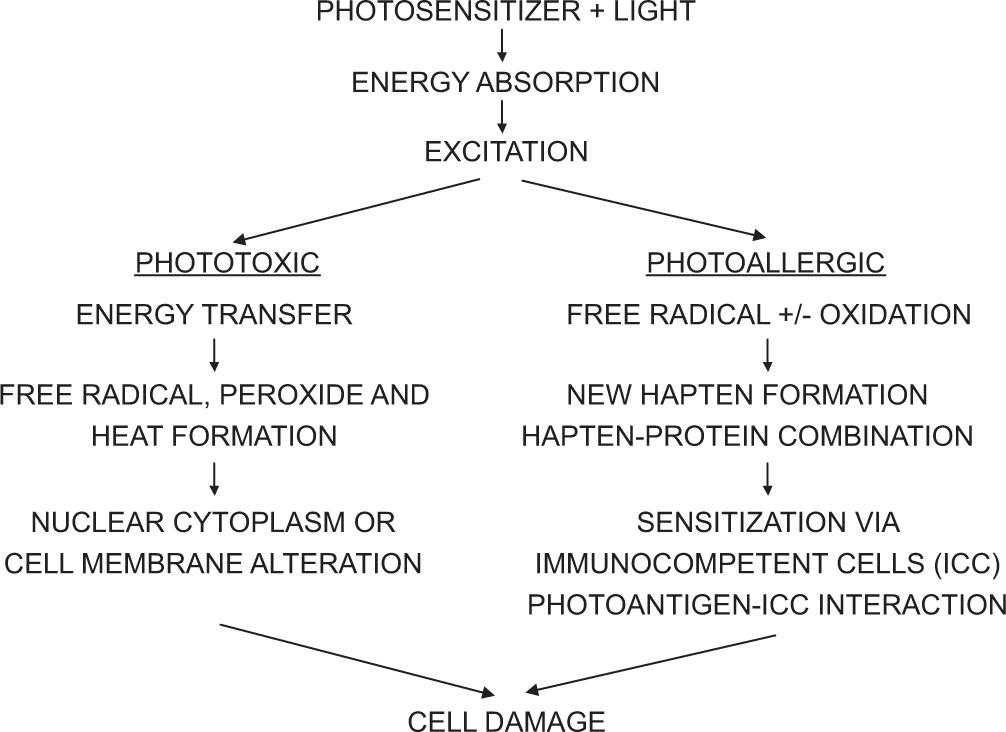
- Mechanism of phototoxicity and photoallergic reactions. ICC: Immunocompetent cells.
Photodynamic reactions
In their excited triplet states, these photodynamic photosensitizers combine with oxygen to produce singlet oxygen or superoxide anions, which can subsequently cause damage to lipids, proteins, nucleic acids, and membranes within cells.[5] The main mechanism underlying the phototoxicity caused by various dyes, coal tar, polycyclic hydrocarbons, anthracene, and porphyrins is photodynamic reactions. Porphyrin photosensitization is also linked to the activation of the conventional complement system. In the porphyrias, this photodynamic process seems to occur largely in the endothelial cells of the superficial dermal vasculature. In addition, phototoxicity brought on by photosensitization to demethylchlortetracycline, chlorothiazide, and chlorpromazine (Thorazine) has been shown to activate complement.
Non-photodynamic reactions
UVB-induced acute sunburn is non-photodynamic in nature and appears to be linked to DNA damage. It is an oxygen-independent process. The psoralens are well-known instances of exogenous, non-photodynamic photosensitizers. One such example is 8-methoxypsoralen, or Oxsoralen. These molecules intercalate with the DNA helix. Depending on the psoralen molecule’s structure, photoactivation with UVA light produces monofunctional and bifunctional adducts with the DNA. In addition, photobinding to proteins and RNA could also occur. Psoralens, however, may also cause photodynamic oxygen-dependent processes. At least, some of the cutaneous reactions in psoralen phototoxicity may be caused by these photodynamic effects, as singlet oxygen production brought on by the combination of several psoralens and UVA has been linked to erythema responses.
Other mechanisms
Cellular damage is the outcome of cutaneous phototoxicity, regardless of the type – photodynamic or nonphotodynamic. In addition to UVB alone, various substances such as psoralens, coal tar extracts, polycyclic hydrocarbons, anthracene, sulfonamides, certain tetracyclines, and chlorpromazine have also been shown to cause photo-induced DNA damage. The in vivo cellular responses, at least in the case of chlorpromazine, seem to be the consequence of membrane damage, and the in vitro interactions with DNA are not seen in the skin. Lysosomal labilization can be brought on by anthracene and porphyrin photosensitivity. In cells, certain tetracyclines can cause photodamage to mitochondria.[6] A sudden urticaria reaction triggered by mast cell degranulation may lead to benoxaprofen photosensitivity. Furthermore, radiation exposure to patients on amitriptyline, chlorpromazine, and chlordiazepoxide can yield harmful photoproducts that could trigger phototoxic reactions.
A metabolite of the parent compound is the photosensitizer in certain photosensitized reactions. It is currently unknown how these manifests as the erythema, edema, and hyperpigmentation typical of phototoxic cutaneous reactions. It appears that these reactions have different erythrogenic mediators. Prostaglandins do not seem to be involved in 8-methoxypsoralen photoreactions, despite their involvement in UVL3-induced erythema. Histamine may act as a mediator in the reactions involving anthracene, benoxaprofen, and hematoporphyrin. In this instance, a lipophilic photoproduct seems to be the cause of mast cell degranulation. It is unclear how these mediators and mechanisms relate to photodamage caused by other photosensitizers.
Action spectrum
The chromophore absorbs these wavelengths, which cause photosensitization. As mentioned, in the absence of exogenous photosensitizers, UVB and less effective UVA can cause phototoxic reactions. Exogenous photosensitizers typically have at least some UVA radiation in their action spectra. Certain chromophores can only be activated by UVB radiation, and the action spectra of some molecules, such as porphyrins and some dyes, include or are limited to visible wavelengths.
Chronic phototoxicity
Years of sun exposure combined with repetitive injury causes distinctive clinical changes such as actinic keratoses, wrinkles, atrophy, hyperpigmentation and hypopigmentation, dilated superficial blood vessels, and the development of nonmelanoma skin cancer.[3] While UVA rays undoubtedly contribute to these changes, the main action spectrum falls in the UVB range. Despite appearing thick, especially on the back of the necks of those with lower skin types (cutis rhomboidalis nuchae), skin that has sustained chronic photodamage is typically fragile, thin, and prone to tearing. Star-shaped or stellate scars form after these tears heal. Numerous hypopigmented macules known as guttate hypomelanosis may arise from damage to melanocytes. Damage to the superficial blood vessels can occur from both direct trauma and inadequate dermal support.
Large areas of actinic purpura may result from this on skin that has been sun-damaged over time. Furthermore, repeated open comedones may result from prolonged sun exposure (Favre Racouchot). The epidermis’ rete ridges may be seen to be both elongated and effaced under a microscope. There is irregular atrophy and hypertrophy, a disorganized arrangement, and a high concentration of aberrant cells. The size, distribution, and tyrosinase content of melanocytes vary, and there seems to be a problem with the pigment’s transfer to keratinocytes.
The most noticeable alteration in the dermis is called actinic elastosis, and it happens gradually. During the first 10 years of life, it can be identified. Increased elastic fiber content appears to be the first alteration, followed by thickening, curling, and more branching. Both UVB and UVA rays are included in the action spectrum for elastosis in experiments. While UVA causes a more delicate elastosis that is positioned deeper, UVB causes a denser elastosis. These alterations seem to result from the different ways that UV radiation penetrates the skin; UVB affects fibroblasts in the upper dermis, while UVA affects fibroblasts in the deeper dermis. Prolonged exposure to infrared radiation can cause alterations in the skin that are similar to those caused by UVB radiation, such as the development of precancerous and cancerous lesions. Exogenous photosensitizers are generally not linked to long-term cutaneous alterations. Nonetheless, research has indicated that certain fluoroquinolones, psoralen compounds, and derivatives of coal tar can cause chronic photocarcinogenesis in laboratory animals. 8-Methoxypsoralen plus UVA radiation (PUVA) photochemotherapy causes human photocarcinogenesis, mainly cutaneous squamous cell carcinomas with a small amount of basal cell carcinomas as well.
According to Stern et al.,[6] psoriatic patients who underwent the Goeckerman regimen – a highly intensive course of treatment that included coal tar and UVB radiation – had a higher incidence of non-melanoma skin cancer. Since coal tar photosensitization occurs in the UVA range, this could indicate an additive carcinogenic effect between the tar and UVB radiation. It should be mentioned, though, that a number of additional investigations were unable to identify the Goeckerman therapy’s photo carcinogenic effects. Chronic alterations in the skin can also result from porphyrin molecule photosensitization. In the absence of aberrant porphyrin levels, a low-grade phototoxic response to several medications has resulted in blisters, skin fragility, milia formation, and scarring. Nalidixic acid, furosemide, tetracycline hydrochloride, sulfones, naproxen, and amiodarone are some of these medications. In addition, prolonged exposure to solarium alone has been shown to cause the same cutaneous changes. Regarding these agents, the light, fluorescent, and electron-microscopic observations are the same as those related to the cutaneous porphyrias. As a result, these responses are known as pseudoporphyria. As with cutaneous porphyrias, it appears likely that the endothelial cells of the upper dermal vasculature are the primary sites of phototoxic injury.
OTHER PHOTOTOXIC REACTIONS
Lichenoid and lichen planus like reaction
Clinically, these reactions can take the form of violaceous papules with Wickham’s striae or scaling violaceous erythema. They might be identical to idiopathic lichen planus both morphologically and histologically. They do not affect the oral mucosa, in contrast to idiopathic lichen planus, and they are found in sun-exposed areas. Such reactions have been reported to be induced by a number of medications, including demethylchlortetracycline, quinine, hydroxychloroquine, hydrochlorothiazide, and chloroquine. Although the exact mechanism behind this reaction is unknown, phototoxicity seems to be the likely culprit.
Photo-onycholysis
Many medications taken systemically have been shown to cause photo-onycholysis. Tetracyclines, psoralens, benoxaprofen, fluoroquinolones, and chloramphenicol are a few of these.[7] It usually affects the distal third of the nail and is tender. After at least two weeks of treatment, it usually happens. There is still much to speculate about regarding the mechanism of this phototoxic response.
PHOTOALLERGY
Photoallergy comes in two varieties. These could be delayed papular, vesicular, or eczematous reactions [Figure 3], or they could be immediate urticarial reactions. They signify an acquired altered reactivity in both cases, which can be attributed to either an immediate antigen antibody response or a delayed cell-mediated hypersensitivity process. A photoallergic reaction typically requires less energy to produce than a phototoxic reaction. Exogenous agent-induced photoallergy almost always manifests as a delayed hypersensitivity reaction [Figure 2].
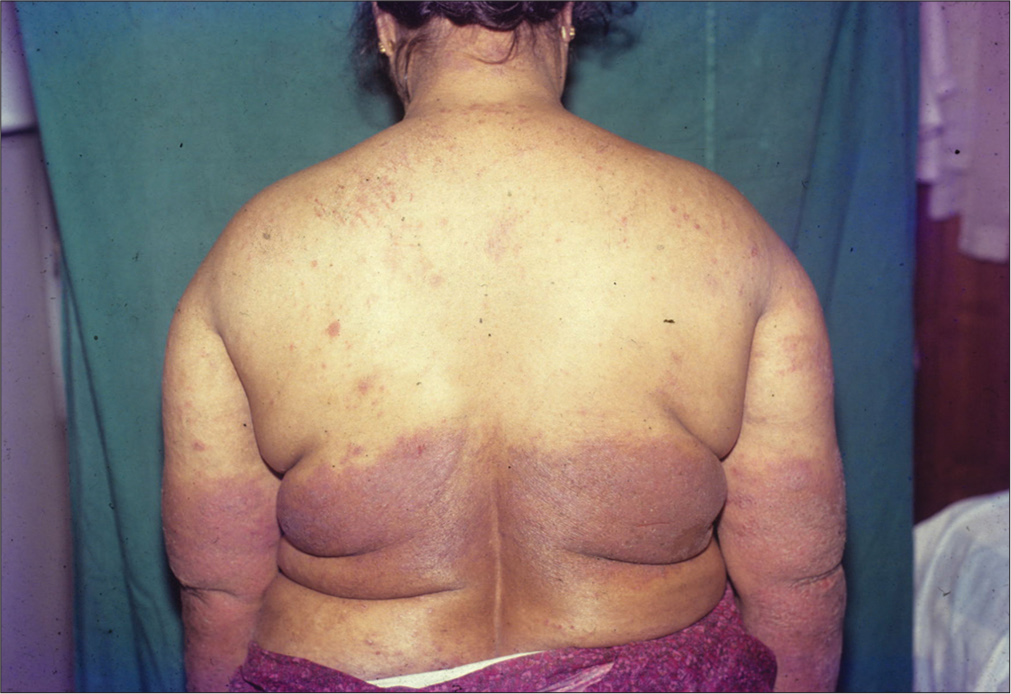
- A case of photoallergic dermatitis in a 50-year-old woman.
The acquired nature of the process, the definition of reaction times and clinical features, flares of previously involved sites following irradiation of distant areas, and the demonstration of passive transfer and reverse passive transfer to normal recipients using the patients’ serum or plasma are other features that can occasionally be determined.
The acute wheal and flare response are histologically linked to edema and vasodilatation. The vascular walls’ increased permeability is one of the first alterations. In the early hours following the challenge, an infiltrate containing neutrophils or eosinophils may be observed. In addition, eosinophil granule protein may be present in the dermis. If the morphological process in the delayed hypersensitivity reaction is eczematous, vesiculation and epidermal spongiosis may be observed. A dense perivascular round cell infiltrate in the dermis, similar to that observed in allergic contact dermatitis, is the characteristic finding.
Horio’s report of both an immediate urticarial reaction and a delayed reaction to chlorpromazine stands out as an anomaly.[8] Histologically, dense perivascular lymphocytic infiltrates in the dermis, akin to those observed in delayed cell-mediated hypersensitivity response in general, are indicative of delayed reactions. Similar to drug-induced phototoxicity, UVA rays are typically at least part of the action spectrum; however, UVB and possibly visible rays are the wavelengths that cause an elicitation. The majority of the knowledge regarding the immunological components of these reactions comes from research on contact photoallergy. The available data are typically insufficient to fully understand the pathophysiology of systemic drug-induced photoreactions, even though some of them are histologically and clinically suggestive of an allergic process.
Topical medications are typically the most frequent cause of skin lesions in cases of photoallergy. In daily practice, topical antimicrobials, topical sunscreen products, and topical non-steroidal anti-inflammatory drugs are most commonly used.[9] Conversely, systemic agents occur far less frequently.
TOPICAL AGENT INDUCED PHOTOALLERGY
Contact photoallergic reactions share no clinical or histological characteristics with other forms of allergic contact dermatitis. Morphologically, they are typically eczematous, though they can also have a severe vesiculobullous pattern or just a simple erythema. Scratching and rubbing may cause lichenification. Histologically, bulla and vesicles can appear, but epidermal spongiosis is typically seen. A perivascular lymphocytic infiltrate is visible in the dermis.
As mentioned, the UVA rays are typically at least included in the action spectrum. In populations exposed to potential photocontactants, these reactions happen far more frequently in adults. For instance, health-care personnel in mental health facilities are more likely to develop a photoallergic reaction to chlorpromazine. Usually, the eruption is limited to areas that are exposed to the sun and photocontactant. However, autoeczematization reactions and conditioned irritability can lead to generalization.
Sunscreen products are the most common cause of photoallergy, particularly in the US and Europe. UV filters are the main source of photoallergic dermatitis, particularly benzophenone-3.[10]
Antimicrobial agents are also a fairly frequent cause. Nonsteroidal anti-inflammatory drugs are the main class of topical medications that cause photoallergy. Similar to phototoxicity, long-term exposure to chlorpromazine, dioxopromethazine, ketoprofen, musk ambrette, halogenated salicylanilides, and quinidine combined with persistent UV radiation can also result in chronic actinic dermatitis.
SYSTEMIC AGENT INDUCED PHOTOALLERGY
As mentioned, the majority of these systemic agents’ reactions are phototoxic. Due to the eczematous nature of the reaction and the existence of positive photopatch tests, some reactions have been assumed to be photoallergic. It is dubious how these tests should be interpreted because these substances are all phototoxins. Nonetheless, it appeared that a small number of documented photoreactions to phenothiazines, calcium cyclamate, thiazide diuretics, sulfonamides, and sulfonylurea antidiabetic medications were photoallergic. In addition, it seemed that the infrequent diphenhydramine reactions with UVB radiation were photoallergic. There have been reports of both transient and persistent photosensitivity reactions to quinidine and hydrochlorothiazide. For at least three years, demethylchlortetracycline caused persistent photosensitivity. In 2% to –3% of patients, the non-steroidal anti-inflammatory drug (NSAID) piroxicam causes eczematous reactions in sun-exposed areas; these reactions have been linked to positive photopatch tests, which point to a photoallergic process. It is unclear if this reaction is photoallergic, because it starts a few days after starting the medication.
Photosensitivity in children
The majority of pediatric photosensitive illnesses are either genetic or metabolic in nature. If a child exhibits erythema, swelling, or severe pruritus following a brief period of sun exposure, photosensitivity should be suspected. Therefore, obtaining a thorough medical history is crucial when assessing a child who may have a photosensitive disease. It is important to inquire about the age of onset, exposure to possible photosensitizers, and seasonal variation in history. If a child has an underlying photosensitive disorder such as nutritional, metabolic, or genetic disease, the phototoxic and photoallergic reactions become more severe. Photopatch and phototesting in children are challenging procedures to carry out. Managing photosensitivity in children requires both sun protection and avoidance. It is recommended that these children and their parents receive adequate counseling regarding the illness and different photoprotective techniques.
Differential diagnoses of phototoxicity and photoallergy
Differential diagnoses of photosensitivity can include any kind of non-specific dermatitis [Table 2]. Nasolabial folds and eyelids are among the skin folds that are most commonly affected by allergic contact dermatitis brought on by inhaled allergens. Since these regions receive very little UV radiation, photo-induced reactions are not anticipated to occur there. Both irritant contact dermatitis and allergic contact dermatitis are primarily seen in areas that are either sun-exposed or sun-protected and where there is direct contact with the inhalant or contact allergen.
| MED A | MED B | Visible light | |
|---|---|---|---|
| Phototoxicity | |||
| 1. Irritant contact dermatitis | |||
| Mostly seen in locations where the irritant comes into direct contact with the skin, including sun-exposed or sun-protected areas | -- | -- | -- |
| Photoallergy | |||
| 1. Allergic contact dermatitis | |||
| Resulting from allergens inhaled primarily affects eyelids and nasolabial folds, among other skin folds which are spared in photo-induced reactions | -- | -- | -- |
| 2. Chronic actinic dermatitis | |||
| Photoexposed skin, especially on the face, back of the neck, forearms, and dorsal hands, presents with itchy, lichenified eruptions that are often distinguished from non-photoexposed regions by a distinct line. | Low or normal | Low or normal | Low or normal |
| Both | |||
| 1. Polymorphous light eruption | |||
| Pruritic papules or plaques seen at sun exposed area resolving in a few days | Normal | Normal | Normal |
| 2. Solar urticaria | |||
| Morphologically resembling pruritic urticarial plaques, usually appearing minutes after sun exposure, and ultimately going away in a few hours | Normal | Normal | Normal |
MED: Minimal erythema dose
Approaching patients with phototoxicity and photoallergy
A thorough history that covers the relationship between eruption and sun exposure, the duration of lesions, exposure to photosensitizing agents, family history, age of onset, seasonal variation, and systemic symptoms is essential when evaluating patients with photosensitivity.
An investigation into the morphology and distribution through physical examination, with particular focus on the presence of symptoms on the head, face, neck, arms, legs, and torso, and their absence below the lips, chin, nasolabial folds, and post-auricular area may offer more hints for diagnosis. Advanced diagnostic techniques include skin biopsies, phototesting, photopatch testing, antinuclear antibody titers, and porphyrin levels.
When systemic photosensitizers are used, extensive eruption is typically seen. However, lesions from topical photosensitizers are found in areas exposed to UV light and the photosensitizer chemical. While eczematous eruptions with pruritus typically indicate photoallergy, vesicular and bullous lesions with severe symptoms and complaints of a burning sensation are likely indicative of phototoxicity [Table 1].
A skin biopsy might be required in a few uncommon cases to distinguish between the two dermatoses. Spongiotic dermatitis is linked to photoallergy, while necrotic keratinocytes are a hallmark of phototoxicity [Table 1].
In the assessment and diagnosis of patients with known photosensitivity, phototests and photopatch tests are also crucial, particularly when a physical examination and medical history are insufficient to reach a conclusion or identify the underlying cause. The MED should be identified prior to phototesting and photopatch testing. On the body surface, ideally the back, duplicate sets of photoallergens are applied symmetrically and covered with opaque tape. Subsequently, increasing dosages of UVA radiation are applied, and MEDs are added. The MED for UVA and UVB is the amount of radiation that causes noticeable erythema to cover the whole irradiated region.[1] When it comes to phototoxicity and photoallergy, the MED for UVA radiation is lower than the general population.[11]
Testing with photopatch is not advised when there is possible phototoxicity. However, the percentage of patients who receive positive results that are clinically relevant is only 10%.[12] When drugs are administered enterally or parenterally, photopatch tests frequently yield negative results for photoallergy because the occurrence of the cutaneous lesions is actually caused by a particular metabolite rather than the medication itself.
Management of phototoxicity and photoallergy
The optimal course of action should be determined first to prevent the phototoxic or photoallergic agent that is causing the problem. Additionally, avoiding UV radiation is crucial, and sunscreens with effective UVA filters should be used on a regular basis.
Topical corticosteroids are the recommended medication for the symptomatic management of acute reactions. Antihistamines might be beneficial as well. Systemic corticosteroids may also be used if there are severe reactions. For acute and severe exacerbations, a brief course of treatment (prednisone 1 mg/kg) can be started within a week or two.[11] If there is noticeable hyperpigmentation following the resolution of the acute event, depigmentation techniques may be employed. One option is to try laser therapy or a combination of 5% hydroquinone, 1% hydrocortisone, and 0.1% retinoic acid.
Psoralen plus UVA, preventive UV phototherapy, or both can be used to treat the majority of photodermatoses. One of the fundamental components of prophylactic phototherapy in photosensitive individuals is the “hardening” phenomenon. If there is continued significant photosensitivity, the recommended course of treatment would be low-dose broadband or narrowband UVB or phototherapy (PUVA) two to three times/week. It usually takes 15 sessions on average to induce hardening, and the treatment is started in the spring. Patients are instructed to expose themselves to midday sunlight for 15–20 min each week without sunscreen to maintain this hardening state.
Determining which photon wavelength is causing the sensitivity reaction is crucial when selecting the best sunscreen for each patient. This is frequently accomplished by figuring out the minimal erythema dose (MED) of UVA and UVB rays [Figure 4].
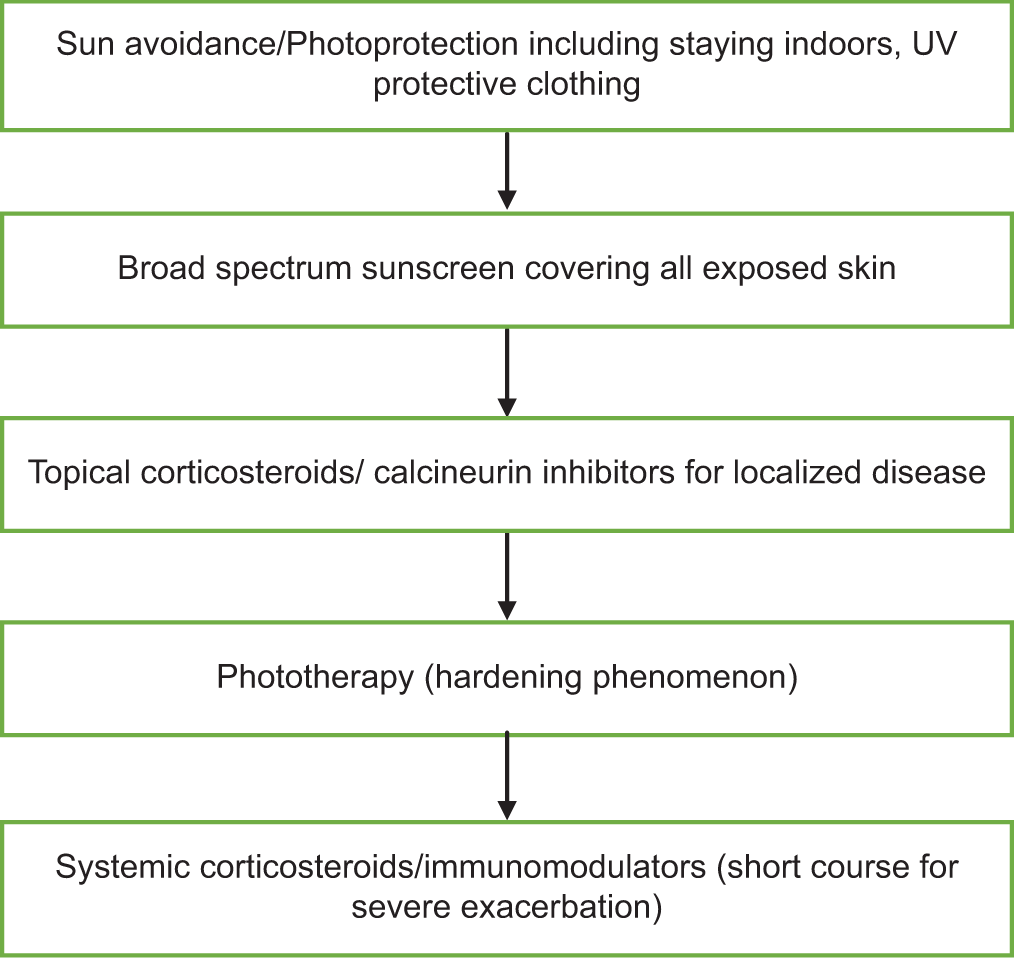
- Treatment ladder in the management of phototoxicity and photoallergy, UV: Ultraviolet.
CONCLUSION
Photosensitivity presents a difficult situation for the patient and the doctor. A methodical approach is essential for the precise diagnosis and management of photosensitivity. Avoiding direct sunlight, sun-tanning areas, photosensitizing chemicals, wearing UV-filtered clothing, using the right sunscreen, and patient education is necessary for reducing the chance of photosensitivity.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Sathish Pai is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Adverse cutaneous reactions to the sun In: Malkinson FD, Pearson RW, eds. Year book of dermatology. Chicago: Year Book Medical Publishers; 1971. p. :5-43.
- [Google Scholar]
- Photodynamic action and diseases caused by light New York: Van Nostrand Reinhold; 1941.
- [Google Scholar]
- Influence of heat, wind and humidity on ultraviolet radiation injury. Natl Cancer Inst Monogr. 1978;50:161-7.
- [Google Scholar]
- Photosensitivity diseases: Principles of diagnosis and treatment (2nd ed). Philadelphia, PA: Saunders; 1988.
- [Google Scholar]
- Skin carcinoma in patients treated with topical tar and artificial ultraviolet radiation. Lancet. 1980;1:732-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous photosensitivity diseases induced by exogenous agents. J Am Acad Dermatol. 1995;33:551-73. quiz 574-6
- [CrossRef] [PubMed] [Google Scholar]
- Chlorpromazine photoallergy: coexistence of immediate and delayed type. Arch Dermatol. 1975;111:1469-71.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic approach to photodermatoses. J Dtsch Dermatol Ges. 2006;4:965-75.
- [CrossRef] [PubMed] [Google Scholar]
- Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- [CrossRef] [PubMed] [Google Scholar]
- Photodermatoses: Classification, evaluation and management. Br J Dermatol. 2009;161:61-8.
- [CrossRef] [PubMed] [Google Scholar]
- Photodermatoses in African Americans: A retrospective analysis of 135 patients over a 7-year period. J Am Acad Dermatol. 2007;57:638-43.
- [CrossRef] [PubMed] [Google Scholar]






