Translate this page into:
Comparative real-world clinical assessment of mometasone furoate 0.1% and fluticasone propionate 0.005% in the treatment of atopic dermatitis
*Corresponding author: Namrata Uttam Mahadkar, Global Medical Affairs, GLENMARK, Mumbai, Maharashtra, India. namratamahadkar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saraf V, Gupta P, Mahadkar NU, Dhoot D, Barkate H. Comparative real-world clinical assessment of mometasone furoate 0.1% and fluticasone propionate 0.005% in the treatment of atopic dermatitis. Indian J Skin Allergy 2022;1:66-8.
Sir,
Atopic dermatitis (AD) is a skin disease with complex genetic background which is chronic and pruritic inflammatory in nature with lifetime prevalence of 20%.[1] Topical corticosteroids (TCS) remain mainstay in treatment in spite of availability of multiple treatment options.[2] TCS are a class of hydrocortisone derivatives with variable potency and having anti-inflammatory as well as anti-pruritic properties but with the side effects profile.[3] In some clinical settings, while least potent corticosteroids may be sufficient, but same medication may not be effective in long-term management.[4] In such situation, mid- to high-potent TCS are favored. Mometasone furoate 0.1% (MF) and fluticasone propionate 0.005% (FP) creams are the two most commonly prescribed TCS, either as once daily (OD) or twice daily (BD) in the management of AD, respectively. Due to dearth of comparative data between these two drugs, this retrospective study was aimed to compare clinical assessment in the treatment of AD.
A retrospective data analysis was done at 186 dermatology clinics across India after obtaining ethics committee approval (Suraksha Ethics Committee Regd No. ECR/644/Inst/ MH/2014/RR-20 Dated August 18, 2021). The data charts were identified by creating a list of patients (age ≥2 years) prescribed either MF or FP cream at all clinics, using the medical record database. Patients with incomplete data were excluded from the study. Effectiveness was assessed by Atopic Dermatitis Severity Index (ADSI)[5] and Investigator Global Assessment (IGA). ADSI (range, 0–15) consists of the sum of the scores for all symptoms such as erythema, pruritus, excoriation, exudation, and lichenification, with scoring on a 4-point scale (range, 0–3). IGA is graded on a 4-point scale (0 being clearance of the signs and symptoms and 4 being severe signs and symptoms). Safety assessment was recorded by number of adverse events (AEs) reported. Results were conferred as mean scores, and Fisher’s exact test and unpaired t-test were used to compare the groups. The level of significance was set at 0.05. Chi-square test was used to analyze the difference in proportion of patients with change in mean scores (based on improvement criteria). Data were analyzed by IBM Statistical Package for the Social Sciences statistics version 20.
Out of 1256 patients, 1007 were considered for final analysis. Entire data were divided into two groups with Group I as MF and Group II as FP. Baseline demographics and study design are depicted in [Table 1 and Figure 1], respectively. The primary objective was to compare the percentage of patients achieving clear/almost clear category of IGA (score of 0 or 1) at week 4 in both the groups. Statistical difference was found in both the groups; however, MF was found to be statistically more significant than FP on intergroup comparison [Table 2]. In addition, in terms of ADSI, both the groups were found to be statistically significant in reducing severity of AD at week 4 compared to baseline with MF statistically more significant than FP [Table 2 and Figure 2]. Both the treatments were safe and well tolerated by patients with no AE related to either of drugs. In all, two patients reported AE in MF (belching and headache) and one in FP (pruritus) with no statistical difference between two.
| Mometasone | Fluticasone | P value | |
|---|---|---|---|
| N | 547 | 460 | |
| Male (%) | 311 (56.85) | 233 (50.65) | |
| Female (%) | 236 (43.15) | 227 (49.35) | |
| Age years (SD) | 21.39±16.09 | 20.005±16.47 | 0.19 |
| Assessment of atopic dermatitis | |||
| Mean atopic dermatitis severity index | 9.49±3.29 | 9.81±3.10 | 0.11 |
| Mean investigator global assessment | 3.09±0.63 | 3.05±0.67 | 0.32 |

- Study design.
| Effectiveness parameter | Group 1 | Group 2 | P-value |
|---|---|---|---|
| No. and percentage of patients achieving clear/almost clear category of IGA | |||
| Week 4 | 528 (96.52) | 429 (93.26) | 0.019 |
| No. and percentage of patients achieving clear/almost clear category of ADSI | |||
| Week 4 | 418 (76.41) | 335 (72.82) | 0.2 |
| Mean ADSI at week 4 | 0.85±1.15 | 1.04±1.45 | 0.02 |
IGA: Investigator’s global assessment, ADSI: Atopic Dermatitis Severity Index
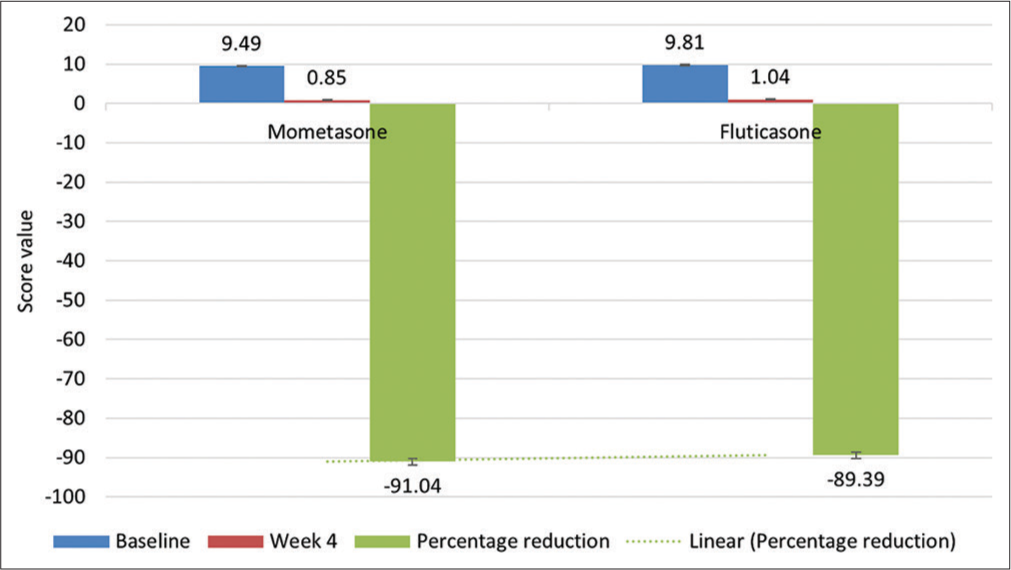
- Mean ADSI at baseline at week 4 and percent reduction in mean ADSI at week 4.
This is the first study comparing effectiveness of MF with FP in AD in India. There are many studies suggesting efficacy, safety, and tolerability of TCS in AD;[6] however, there are limited data about comparison between these two drugs. In our study, MF was found to be statistically significant than FP in IGA and ADSI assessment. In recently published study on contact dermatitis management, MF was found to be statistically significant than FP.[3] In some previous studies, both the molecules were found to better than other TCS of the same or lower potency[6] but with no comparative study between these two. Although both the molecules fall under mid-potency group due to possibility of better anti-inflammatory and anti-pruritic actions, mometasone is potentially effective.[7] Another well-established advantage of MF over other TCS is its once-daily application to achieve the clinical effect which indirectly help to increase the patient compliance and may reduce the cost of therapy. Multiple studies have documented the better patient compliance with once-daily versus twice-daily treatment regimen.[8]
To the best of our knowledge, the present study is the first real-world comparison between mometasone furoate 0.1% and fluticasone propionate 0.005% in AD. The limitation of this study lies with its retrospective design. In conclusion, both the molecules were found to be effective and safe in the management of AD. However, mometasone furoate 0.1% was found to have better effectiveness than fluticasone propionate 0.005% in AD. Looking at the advantage of mometasone, it can be considered as early option in armamentarium of AD management.
Acknowledgment
Authors would like to acknowledge dermatologists from India for sharing clinical data for conduct of this study.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Dhiraj Dhoot, Dr. Namrata Mahadkar, and Dr. Hanmant Barkate are employees of Glenmark Pharmaceuticals Ltd., India.
References
- Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Arch Dermatol. 1998;134:805-9.
- [CrossRef] [PubMed] [Google Scholar]
- Topical corticosteroids in atopic dermatitis. BMJ. 2003;327:942-3.
- [CrossRef] [PubMed] [Google Scholar]
- A real world retrospective analysis of comparison of effectiveness and safety of mometasone furoate and fluticasone propionate in the management of eczema and dermatitis. IP Indian J Clin Exp Dermatol. 2021;7:120-4.
- [CrossRef] [Google Scholar]
- Mometasone Furoate: A well-established topical corticosteroid now with improved galenic formulations. J Clin Exp Dermatol Res. 2013;4:184.
- [Google Scholar]
- Standardized grading of subjects for clinical research studies in atopic dermatitis: workshop report. Acta Derm Venereol Suppl (Stockh). 1989;144:28-30.
- [Google Scholar]
- Use of topical corticosteroids in dermatology: An evidence-based approach. Indian J Dermatol. 2017;62:237-50.
- [CrossRef] [Google Scholar]
- A simple RP-HPLC method for the simultaneous quantitation of chlorocresol, mometasone furoate, and fusidic acid in creams. J Chromatogr Sci. 2009;47:178-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of patient compliance with once-daily and twice-daily antibiotic regimens in respiratory tract infections: Results of a randomized trial. J Antimicrob Chemother. 2007;59:531-6.
- [CrossRef] [PubMed] [Google Scholar]





