Translate this page into:
Polymorphous light eruptions
*Corresponding author: Reena Rai, Department of Dermatology, PSG Hospitals, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India. drreena_rai@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Rai R, Srinivasan H. Polymorphous light eruptions. Indian J Skin Allergy. 2024;3:36-40. doi: 10.25259/IJSA_8_2024
Abstract
Polymorphous light eruption (PMLE) is the most common, immunologically acquired photo-dermatosis due to delayed hypersensitivity reaction to sunlight. It presents with recurrent, pruritic, non-scarring lesions of distinct morphology affecting the sun-exposed body parts. This review article focuses on immunopathogenesis, clinical features, and treatment option of PMLE.
Keywords
Polymorphous light eruption
UV radiation
photoprotection
INTRODUCTION
Polymorphous light eruption (PMLE) is the most common, immunologically acquired photo-dermatosis due to delayed hypersensitivity reaction to sunlight. PMLE most commonly affects females in the second– third decade of life and usually presents with recurrent, pruritic, non-scarring lesions of distinct morphology affecting the sun-exposed body parts.[1] It is more common in temperate areas than in tropical places. The spectrum of light involved is ultraviolet A (UVA), ultraviolet B (UVB), and visible light [Figure 1]. The prevalence may depend on the latitude and amount of UVA and UVB in these regions.[2] Genetic susceptibility and environmental exposure play an important role in the pathogenesis of PMLE.[3] There is a strong positive family history and familial clustering of PMLE among first-degree relatives, with a prevalence of 21% and 18% in monozygotic and dizygotic twins, respectively.[1,3] The familial type of PMLE is inherited through an autosomal dominant mode with human leukocyte antigen (HLA) associations.[2] This review article focuses on immunopathogenesis, clinical features, and treatment options involved in PMLE.

- Spectrum of light.
PATHOGENESIS
The exact etiopathogenesis of polymorphous light eruption (PMLE) is still not well understood, but the disease is linked to a delayed-type (type IV) hypersensitivity reaction to one or more endogenous photo-antigens. In allergic contact dermatitis, interleukin (IL)-1 family of cytokines, especially IL-36 alpha and gamma, are increased, which is also observed in patients with PMLE. These cytokines activate the downstream signaling pathway, such as nuclear factor kappa-B (NF-KB), mitogen activated protein kinase (MAPK) pathways, and promote further inflammatory cascade.[4] IL-36 also has the ability to increase the innate immune response by activating toll-like receptor-3 (TLR-3) and raises the levels of antimicrobial peptides (AMPs) in the skin. Dysregulation of AMPs is found in skin lesions of PMLE, which triggers the release of IL-31 from keratinocytes and is an important cytokine in the pathogenesis of pruritic lesions in these patients.
In normal individuals, ultraviolet radiation (UVR) causes immunosuppression through activation of regulatory Treg cells (T cells), and recruitment of IL-4-positive neutrophils into normal human skin. After UV irradiation, there is a release of immunosuppressive cytokines (tumor necrosis factor-alpha [TNF-α], IL-4, and IL-10), infiltration of macrophage, and migration of Langerhans cells from the epidermis to draining lymph nodes. In PMLE, failure of this UV-induced immunosuppression leads to reduced production of neutrophil-derived TNF-α, IL-4, and IL-10, failure of Langerhans cell migration, and lack of neutrophil infiltration [Figure 2].
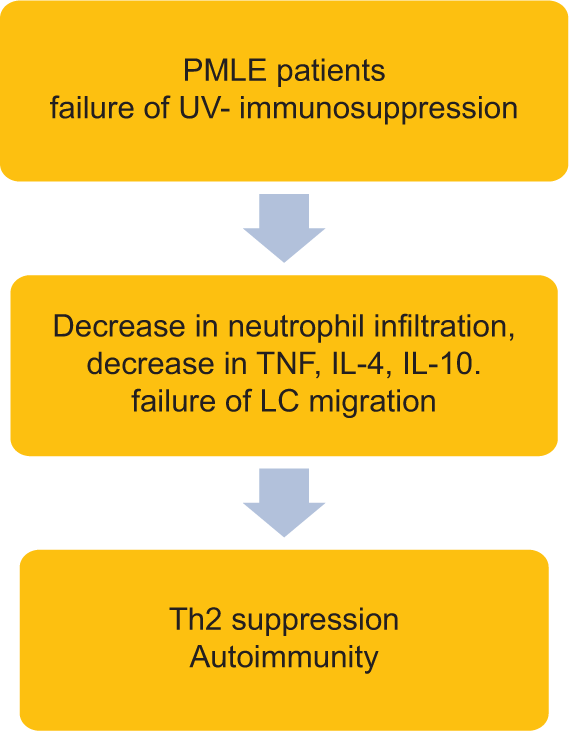
- Failure of ultraviolet induced immunosuppression. LC: Langerhans cells, IL: Interleukin, PMLE: Polymorphous light eruption, TNF: Tumor necrosis factor.
Normally, the immune response is kept in check by mast cell-derived cytokines such as IL-10 (anti-inflammatory cytokine) and recruitment of CD4+CD25+FoxP3 T cells, which is crucial for induction of immune tolerance and control of inflammation. In patients with PMLE, these T cells are decreased, leading to a loss of immune tolerance resulting in autoimmunity [Figure 2].
Certain genes in normal skin such as complement 1s subunit, scavenger receptor-B1 (SCARB-1), caspase-1 (CASP-1) and fibronectin (FN-1)-1 are associated with apoptotic cell clearance. In patients of PMLE, reduced expression of these genes may lead to partial failure of apoptosis, leading to the accumulation of apoptotic cells, which acts as a potential trigger for the formation of autoantigens, thereby promoting the disease process.
Vitamin D3 and its analogs have an immunotherapeutic and photoprotective effect on the skin. The immunosuppressive function is by induction of T cells, activation of mast cells to release anti-inflammatory cytokines like IL-10, and anti-inflammatory effect by modulating the level of cutaneous AMPs. Low levels of Vitamin D3 are found in PMLE as compared to normal individuals, which may be due to sunlight avoidance among patients with photosensitive skin. A deficiency of Vitamin D3 is considered a risk factor to increase disease susceptibility by impairing immune function and increasing inflammation. PMLE being more in females is possibly due to the hormone 17β-estradiol, which prevents the UVR-induced immunosuppression and inhibits the release of immunosuppressive cytokines (IL-10) from keratinocytes, thus triggering the inflammation.[2,3]
CLINICAL FEATURES
The most common UVR-causing skin lesions are UVA (320–400 nm) and less commonly by UVB. The lesions are polymorphic and can present as papules (including solar purpura), papulovesicular, plaques, erythema multiforme-like, prurigo-like or insect bite (strophulus)-like lesions, and rarely hemorrhagic papules (purpura).[3] The areas commonly involved are the upper chest, “V” area of the neck, upper arms, forearms, back of hands, thighs, and the sides of the face.[1] Prolonged sun exposure can also lead to a natural hardening. Juvenile spring eruption is a localized variant of PMLE affecting young boys of 5–15 years. It presents as small papules or papulo-vesicles on the helix of the ears. The other rare variants of PMLE include benign summer light eruption (a mild variant of PMLE), solar purpura (lesions predominantly affecting legs), lichen planus-like, erythema multiforme-like, and PMLE sine eruption (intense pruritus in sun-exposed parts without visible lesions).[5,6]
PHOTO TESTING AND PHOTO PROVOCATION TEST
Photo testing analyses the skin’s sensitivity to UVR by determining the minimal erythema dose (MED). It is the UVR dose required to elicit perceptible erythema 24 h after administration.[7] The most common light source used for MED testing is the solar simulator containing Xenon arc with filters. Wavelengths of UVR commonly used in photo testing are 300–305 nm and 360–370 nm. The sites commonly preferred are the back, gluteal region, or inner aspect of the forearm. MED is determined by exposing patient’s back to UVR (six adjacent areas of 1 cm2 each over patient’s back) and the reading is done 24 h post-irradiation.[8]
After MED determination, the photo-provocation test is done by irradiating a larger area of the skin (5 × 8 cm2) with 70% of MED dose. The doses may range from 0.75 to 1.5 MED UVB and 30–50 J/cm2 UVA for three to five consecutive days. The reading is on the day of irradiation and repeated daily up to seven days. (photo-testing) In PMLE, photo provocation test on a previously involved skin shows a positive response to UVA in more than 50% of patients, followed by both UVA/UVB in about 30%, and less common sensitivity is seen to UVB alone.[2]
HISTOPATHOLOGY
Epidermis shows hyperkeratosis/atrophy/spongiosis with or without liquefactive degeneration with dense perivascular lymphocytic infiltrate in the upper and mid dermis. A band-like mononuclear cell infiltration in the upper dermis with subepidermal edema is characteristic of plaque-type PMLE. In erythema multiforme-like lesions, there is prominent dermal edema. Extravasation of erythrocytes in the papillary dermis is seen in a hemorrhagic subtype of PMLE. The rare eczematous form shows parakeratosis, acanthosis, spongiosis, and sporadic dyskeratosis.[2] The histopathological differential diagnoses for PMLE are reticular erythematous mucinosis, Jessener’s lymphocytic infiltrate, lupus erythematosus, and actinic reticuloid the histopathological differential diagnosis is mentioned in table 1.[1,9]
| Cutaneous lupus erythematosus | • More prominent interface changes in the epidermis and peri-adnexal • Presence of apoptotic keratinocytes • Absent papillary edema • Increased dermal mucin • Numerous CD123-positive plasmacytoid dendritic cells |
| Jessener’s lymphocytic infiltrate | • Absent epidermal change • Absent papillary dermal edema • More prominent dermal mononuclear infiltrate |
| Actinic prurigo | • Epidermal hyperplasia • More prominent lymphocyte spongiosis and exocytosis • Conspicuous multinucleated giant cells • Large atypical lymphocytes |
| Reticulate erythematous mucinosis | • Presence of dermal mucin |
PMLE: Polymorphous light eruption, CD123: Cluster of differentiation 123
DIFFERENTIAL DIAGNOSIS
The important differential diagnosis of PMLE includes the earliest lesions of subacute lupus erythematosus. Non-specific antinuclear antibodies can be positive in PMLE but it should not exceed 1:80 dilution. Actinic prurigo is difficult to differentiate from PMLE due to its overlapping features, but it is present in early childhood. The hypopigmented macular variant of PMLE has to be differentiated from pityriasis alba, pityriasis versicolor, indeterminate leprosy, and nevus anemicus. Eczematous variants of PMLE can mimic allergic contact dermatitis and other photo-dermatosis the clinical differential diagnosis for PMLE is mentioned in table 2.[1,3,10]
| Differential diagnosis | Differentiating features from PMLE |
|---|---|
| Cutaneous lupus erythematosus | • Skin lesions can be present in sun-exposed and sun-protected areas • Biopsy and ANA titers help in the differentiation • Presence of vasculitic lesions, malar rash, periungual lesions, and discoid lesions |
| Solar urticaria | • Shorter time of onset of eruption with urticarial plaques that disappear by 1–2 h |
| Actinic prurigo | • Onset in childhood • Heals with scarring |
| Erythema multiforme | • Target lesions • Prominent systemic symptoms (fever, headache, and myalgia) |
PMLE: Polymorphous light eruption, ANA: Antinuclear antibodies.
MANAGEMENT
Management of PMLE includes photoprotective measures, systemic photoprotection, photo-hardening, and topical and oral corticosteroids.
Photoprotective measures are fundamental in managing PLE. It includes the use of protective clothing, hats, and frequent application of sunscreens. Broad-spectrum sunscreens with a high sun protection factor against both UVA and UVB, along with physical sunscreens (zinc oxide and titanium oxide) are effective in preventing PLE eruptions. Sunscreens containing DNA repair enzymes such as photolyase and T4 endonucleases are incorporated into liposomes which enhance the DNA repair and eliminate the UV-induced DNA photo products.[2]
Photoprotective clothing plays an important role in the prevention of PMLE. UPF (UV protection factor) is used as an indicator for UV protective clothing. Closely knitted (smaller yarn-to-yarn spaces) clothing has less fabric porosity, thereby resulting in less transmission of UVR. Dark-colored clothing provides better UV protection by increasing UV absorption. Garments made of polyester and polyester blends have good UV-blocking properties. Patients are instructed to use full hand sleeves, shoes, sunglasses, scarves, and hats with a wider brim to protect the head, ears, neck, sides of the face, other sun-exposed parts, and to avoid tight-fitting clothes.[11]
Studies have found that broad-spectrum sunscreens with 1% ectoin may be effective in preventing skin eruptions of PMLE. Ectoin is a multifunctional natural active osmolyte extracted from halophilic bacteria, which protects Langerhans cells from UV damage and UVA-induced stress response in human keratinocytes.[12] Patients should also be counseled about the proper method and frequency of application of sunscreen by following the “teaspoon rule” (3 mL each for face, arms, and 6 mL each for trunk and legs).[1]
Systemic photoprotection: Oral antioxidants act as a free radical scavenger which can be used in the prevention of PMLE. Oral beta carotene at a dose of 75–100 mg/kg/day and topical tocopheryl acetate can act as an adjuvant treatment in preventing PMLE.[2] Polypodium leucotomos extract (PLE) can be used as a topical or systemic photoprotector. It is a natural substance with antioxidant properties. The proposed mechanism of action includes DNA repair ability and prevention of immune cell depletion upon UV irradiation.[13] Afamelanotide, an α-melanocyte-stimulating hormone analog induces skin pigmentation, provides photoprotection, antioxidant activity, and enhances DNA repair.[2]
Natural photo-hardening with gradual exposure to sunlight can prevent the eruption of skin lesions in milder variants of PMLE. Phototherapy can be used to produce a natural hardening effect without provoking skin eruptions. It increases melanin production, thickens the stratum corneum, depletes neoantigens and epidermal Langerhans cells, normalizes the influx of neutrophils, and increases the serum Vitamin D3 levels. Suberythemal doses of broad and narrow band UVB, psoralen with UVA phototherapy are administered 2–3 times/week for six weeks which can induce immunosuppression lasting for 4–6 weeks. Exacerbations of skin lesions may occur during the early course of phototherapy for which a potent topical or short course of systemic steroids may be required.
Others: Vitamin D3 and its analogs (calcipotriol) have preventive and therapeutic effects in PMLE by immunosuppressive properties. It also provides protection from UV-induced DNA damage and improves DNA repair. In patients with a milder form of PMLE, a short course of topical potent steroids is used on affected areas for 5–7 days to reduce inflammation, and oral antihistamines are used to reduce itching.
In patients with a severe form of PMLE, a short course of oral corticosteroids is given for 4–5 days. Steroid-sparing drugs such as oral azathioprine (50–100 mg/day) and cyclosporine (3–4 mg/kg/day) can be used in severe and refractory cases. Small randomized studies have shown improvement with the use of antimalarials such as hydroxychloroquine in PMLE. Nemolizumab is an anti-IL-31 monoclonal antibody used in chronic pruritic conditions that can be given in pruritic lesions of PLE.[2]
PMLE is a persistent disease thar often improves over time (years) and may resolve in some patients. Persistence of PLE lesions for a longer time may precede the development of lupus erythematosus.[14] Females with prolonged PMLE have a tendency to develop other autoimmune diseases and thyroid dysfunction.[15]
CONCLUSION
Polymorphous light eruption is common photodermatoses with variable clinical presentation. It has to be differentiated from its close mimics by proper clinical history and distribution of skin lesions. A good knowledge of the molecular mechanisms and immunopathogenesis of PMLE may help to develop novel therapeutic strategies to target the specific cytokines and inflammatory mediators. Appropriate therapy helps in prevention and treatment improving the quality of life.
Take home points
Polymorphous light eruption (PMLE) is one of the common, immunologically mediated photo-dermatosis due to delayed hypersensitivity reaction to sunlight
Genetic susceptibility (strong family history, familial clustering, autosomal dominant inheritance, and HLA susceptibility) and environmental factors (UVR and temperate climate) play an important role in PMLE
The newer concepts in the immunopathogenesis of PMLE include loss of UV-induced immunosuppression, involvement of the IL-1 family of cytokines, activation of toll-like receptors, and dysregulation in the levels of antimicrobial peptides
The skin lesions of PMLE can be polymorphic involving the upper chest, “V” area of the neck, upper arms, forearms, back of hands, thighs, and the sides of the face
The principles in preventing PMLE include counseling about photoprotective measures and proper use and application of sunscreens
Understanding the molecular genetics and immunopathogenesis of PMLE helps in developing novel therapeutics for targeted therapy.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Polymorphous light eruption-an Indian scenario. Indian Dermatol Online J. 2021;12:211-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunopathogenesis and management of polymorphic light eruption. Dermatol Ther. 2021;34:e15167.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphic light eruption: What's new in pathogenesis and management. Front Med (Lausanne). 2018;5:252.
- [CrossRef] [PubMed] [Google Scholar]
- New insights into the mechanisms of polymorphic light eruption: Resistance to ultraviolet radiation-induced immune suppression as an aetiological factor. Exp Dermatol. 2009;18:350-6.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile spring eruption among soldiers: A report of a large outbreak. Australas J Dermatol. 2021;62:e265-6.
- [CrossRef] [Google Scholar]
- Polymorphous light eruption: Clinic aspects and pathogenesis. Dermatol Clin. 2014;32:315-34.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal erythema response (MED) to solar simulated irradiation in normal Indian skin. Indian J Dermatol Venereol Leprol. 2004;70:277-9.
- [Google Scholar]
- Equipment developed for simplifying routine phototesting in dermatology. Photochem Photobiol Sci. 2023;22:2907-17.
- [CrossRef] [PubMed] [Google Scholar]
- The Photosensitivity Disorders. 2016. Plastic surgery key. Available from: https://plasticsurgerykey.com/the-photosensitivity-disorders/ [Last accessed on 2016 Jul 31]
- [Google Scholar]
- Craig A Elmets, MD. 2023. Polymorphous light eruption. Available from: https://www.uptodate.com/contents/polymorphous-light-eruption [Last accessed on 2023 Feb 23]
- [Google Scholar]
- Role of clothes in sun protection In: Cancers of the skin: Proceedings of the 8th world congress. Berlin, Heidelberg: Springer Berlin Heidelberg; 2002. p. :15-25.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of polymorphic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-13.
- [CrossRef] [PubMed] [Google Scholar]
- The combination of oral and topical photoprotection with a standardized Polypodium leucotomos extract is beneficial against actinic keratosis. Photodermatol Photoimmunol Photomed. 2023;39:384-91.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term course of polymorphic light eruption: A registry analysis. Front Med (Lausanne). 2021;8:694281.
- [CrossRef] [PubMed] [Google Scholar]
- Disease associations in polymorphous light eruption: A long-term follow-up study of 94 patients. Arch Dermatol. 1998;134:1081-5.
- [CrossRef] [PubMed] [Google Scholar]






