Translate this page into:
Pigmented contact dermatitis: An updated review
*Corresponding author: Yasmeen Jabeen Bhat, Department of Dermatology, Venereology and Leprosy, Government Medical College, Srinagar, Jammu and Kashmir, India. yasmeenasif76@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhat YJ, Ul Islam M, Sultan S. Pigmented contact dermatitis: An updated review. Indian J Skin Allergy. 2024;3:12-20. doi: 10.25259/IJSA_44_2023
Abstract
Pigmented contact dermatitis (PCD) is a disorder brought on by repeated exposure to low-intensity allergens, usually presenting as blotchy or reticulate slate-gray pigmentation affecting Fitzpatrick skin type IV-VI. The pathogenesis remains unclear; however, type IV hypersensitivity reactions due to allergic sensitization, genetics, ultraviolet exposure, and autoimmunity are to blame. Clinical examination, dermoscopy, patch/photo patch testing, histopathology, and recently, a novel reflectance confocal microscopy and multimodality skin imaging system aid in the diagnosis. Several contact allergens have been linked to PCD, but from an Indian perspective, Kumkum and Paraphenylenediamine are the incriminating agents. Patch testing plays an immense role whenever PCD is diagnosed, primarily due to contact allergens. Devastating psychological impacts can result from PCD-related deformity on social acceptance, mental health, and self-esteem. Avoiding allergens, wearing broad-spectrum sunscreen, and engaging in sun-protective behavior are general measures for treating the condition.
Keywords
Non-eczematous
Pigmented contact
Riehl melanosis
Allergen
Patch test
Treatment
Laser
INTRODUCTION
Pigmented contact dermatitis (PCD) (Synonyms: Riehl’s Melanosis/Pigmented cosmetic dermatitis) is defined as an eczematous type of contact dermatitis brought on due to repeated exposure to low-intensity allergens in cosmetic products and fragrances. It is more common in women in their mid-to late-forties. Clinically presents as diffuse facial melanosis in the form of reticulate or blotchy slate-gray to brown hyperpigmented macules over the face and neck of skin type (Fitzpatrick IV–VI).[1] This pigmentation is more noticeable over the lateral aspect of the face than on the central part and is frequently accompanied by erythema or itching. Diffuse facial melanosis affecting the face and neck area is of great concern and has an immense psychological impact.[2,3] Sarkar et al.[4] reported a Delphi consensus conducted across India and Australia for a uniform nomenclature of lichen planus pigmentosus (LPP) and related entities. Finally, an 80% consensus concluded to frame an umbrella term acquired dermal macular hyperpigmentation (ADMH), of which PCD was one of the diseases fulfilling the criteria and others being LPP and Erythema dyschromicum perstans/Ashy dermatosis. It was also agreed that ADMH should be considered as a broad entity governing both with and without contact sensitization. There is considerable overlap of symptoms among the diseases that come under the umbrella of ADMH.[2,4] As a result, a review of clinical aspects reaffirmed by dermoscopy, patch testing, and histopathology can help to improve understanding of the disease and its management.
A BRIEF HISTORY AND EVOLUTION IN NOMENCLATURE
It was Riehl in 1917 who first described Riehl’s Melanosis (RM). He speculated that wartime rationing and contaminated food were to blame.[1,5] Following the Second World War, cosmetic use increased considerably, leading to increased facial pigmentation cases.[6] Hoffmann and Habermann reported similar cases secondary to certain oils and hydrocarbons. They named it “Melanodermatitis Toxica.”[7] By the end of 1960, Minami and Noma coined the term “melanosis Faciei Feminae” and thought a makeup allergy caused it.[8] In 1970, Osmundsen first used the term “pigmented contact dermatitis” after identifying several instances of unusual facial hyperpigmentation in the Copenhagen area.[9] In 1973, Nakayama was the first to report pigmented cosmetic dermatitis and postulated it as a new disease.[10]
Over the years since PCD came to light, most experts have considered it synonymous with RM of the face, with the etiology of the condition still being debated. A global consensus has concluded that it should be classified as “pigmented contact dermatitis” instead of RM if the diffuse melanosis involving the cervicofacial region develops secondary to preceding allergic contact. The term “RM” ought to be used if the cause is unknown.[3]
EPIDEMIOLOGY
This condition commonly occurs in women of middle age with dark skin. Initially reported in Japan, other countries such as India, Denmark, South America, France, and South Africa have also reported similar cases. The Consumer Product Safety Commission of the USA and Europe has identified five allergens as potent sensitizers, including paraphenylenediamine (PPD), and has thorough regulations against their use. This may be the reason for the lower incidence of PCD in these developed countries than in developing nations like ours.[5]
PATHOGENESIS AND RECENT MOLECULAR ADVANCES
The possible pathogenesis behind this disease is still unknown, however a few factors have been postulated, including genetic predisposition, autoimmunity, Ultraviolet (UV) radiations, and type IV hypersensitivity.[11] The brief mechanism is described in the flow chart [Figure 1].[2,5,11]
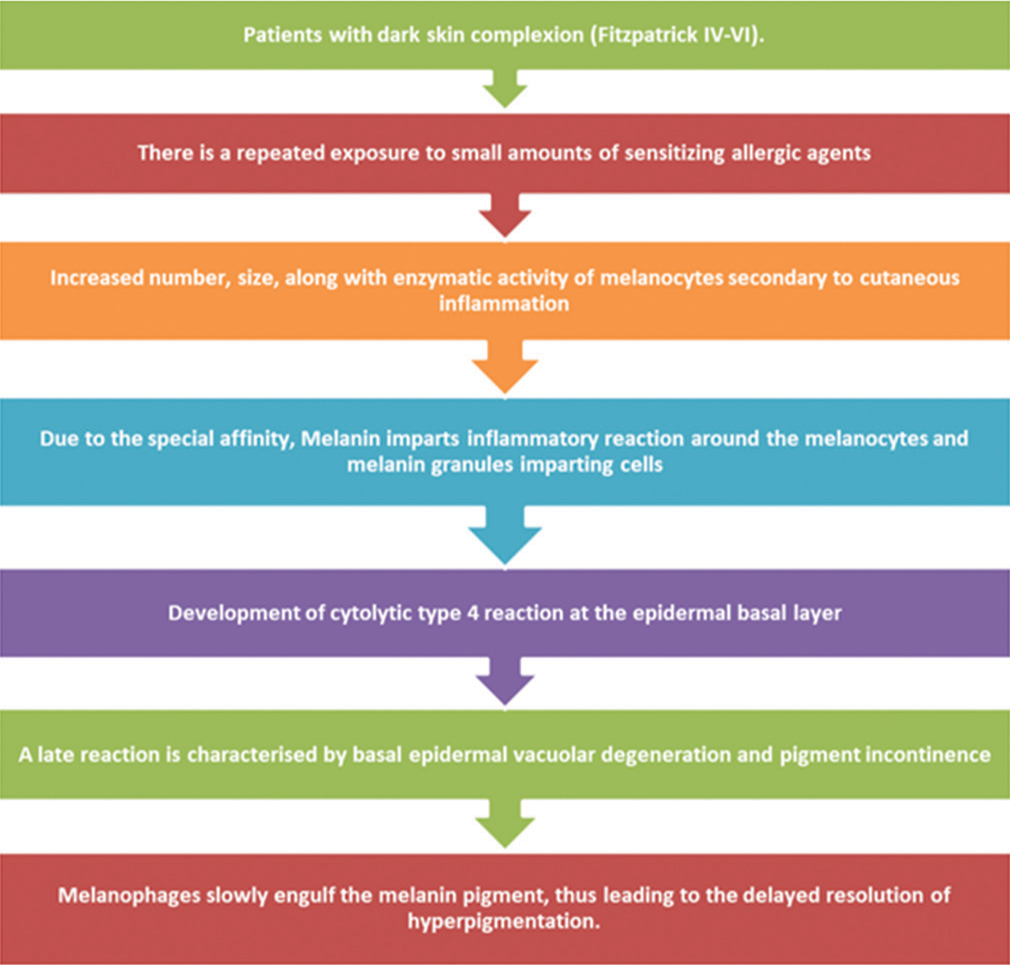
- Flow Chart - Pathomechanism of pigmented contact dermatitis
Recently Jung et al.[12] highlighted the role of guaninedeaminase (GDA) in the pathogenesis of Riehl’s Melanosis (RM). It revealed an increased GDA expression in the lesional skin, which was postulated to promote melanogenesis by the upregulation of stem cell factor (SCF) and endothelin 1 (ET-1), thus proving to be a novel keratinocyte biomarker. Another breakthrough in the molecular pathogenesis was achieved by the study done by Xu et al.[13] They concluded that the fibroblast-derived protein Cellular Communication Network Factor 1 CCNN1 was found to be overexpressed in dermal lesions of RM. It was postulated that Ultraviolet B (UVB) exposures led to the release of CCNN1 from the fibroblasts that induced the melanocytes’ melanogenesis on both transcriptional and translational levels. It was also found that CCNN1 promotes melanogenesis by binding to the integrin α6β1 receptors on melanocytes, that in turn, activates p38 mitogen-activated protein kinase and Extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) signaling pathways, resulting in the activation of microphthalmia-associated transcription factor, tyrosinase-related protein-1, and tyrosinase.
Another study by Woo et al.,[14] in 2020, further evaluated the role of paracrine melanogenic molecules secreted by keratinocytes fibroblasts and endothelial cells in RM. Results revealed an increased expression of Stem cell factor/cluster of differentiation 117-receptor tyrosine kinase (SCF/c-KIT) and Endothelin-1 (ET-1) postulated secondary to UV exposures and subsequent inflammatory processes. Furthermore, an increased number of mast cells has been considered to increase their expressions. Later in 2021, Woo et al.[15] also reported an increased expression of estrogen and progesterone receptors in both epidermal and dermal levels in RM. Thus giving proof of the paracrine role of microenvironmental changes in the vasculature and fibroblasts of the dermis. The increased pigment deposition was postulated due to the increased expression of Keratinocyte growth factor (KGF) from keratinocytes by estrogen, and increased number of infiltrative dermal dendritic cells and regulation of endothelial cells due to progesterone.
ETIOLOGY AND ITS INDIAN POINT OF VIEW
A number of contact allergens have been linked to PCD. However, the etiology remains controversial. It can be either intrinsic or extrinsic and, in most cases, caused by extrinsic agents [Table 1].[5,16-24] In the Indian scenario, we have minimal data on PCD occurrences. In a culturally rich country like ours, Kumkum is a ubiquitous incriminating agent responsible for PCD. Red Kumkum is the only formulation available in the market that can sensitize and cause PCD.[16] Annabathula et al.[17] reported 14 out of 18 patients to have patch test positive for Kumkum. Other suspected contact allergens included turmeric, Sudan-1, and 4-aminoazobenzene reported in four, three, and two patients respectively. Also allergen testing using the Indian standard series was done, and positive results were obtained for nickel fragrances, PPD, cresol, and parthenium in five, two, and one rest of the patients, respectively. Recently, there has been a considerable increase in the use of hair dyes for cosmetic purposes. This has led to skin allergies brought on by hair dye. There can be several culprit allergens in hair dyes, including PPD, toluene-2, 5 diamine, hydrogen peroxide, ammonia, resorcinol, 3 aminophenol, and 4 amino phenol.[5] Bishnoi et al.[18] recently studied 108 patients with ADMH. A positive patch test was seen in 39 (36.1%) patients. Positive patch tests were seen with their own hair colors than with PPD using the Indian standard series.
| Cosmetics allergens | Perfume allergens |
Textiles allergens | Occupational allergens | Medicinal allergens | Others |
|---|---|---|---|---|---|
| Aniline colors | Geraniol oil | Tinopal-CH3566 | Coal tar | Diphenyl-cyclopropenone | Nickel sulfate |
| Red and yellow colors | Lemon oil | Pitch | Kojic acid 1% aqua | Wood dust | |
| Splendid lake red | Musk ambrette | Naphthol AS | Asphalt | Minoxidil | Nickel oxide |
| Chromium hydroxide | Hydroxycitronellal | PPP-HB | Mineral oil | Para tertiary butylphenol formaldehyde | |
| Carbanilides | Benzyl-salicylate | Mercury compound | Chromates | ||
| Ricinoleic | Ylang oil | Formaldehyde | |||
| Hair dyes | Jasmine absolute | Azo colors | |||
| Henna | Lavender oil | Elastic parts | |||
| Kumkum | Canaga oil | ||||
| Sandalwood oil | |||||
| Benzyl alcohol | |||||
| Cinnamic alcohol |
PCD: Pigmented contact dermatitis, PPP-HB: Phosphite polymer of pentaerythritol and hydrogenated bisphenol A
Cosmetic products have been found to cause PCD. A study by Sharma et al.[19] reported 152 positive patch tests secondary to cosmetic allergens. Among the allergens, the most common were preservatives (46.7%) followed by hair dyes (31%), fragrances (16.4%), emulsifiers (3.2%), and vehicles (1.3%). Similarly, Kwong and Lim[20] reported antiperspirant-induced PCD in axilla secondary to limonene, a terpene derived from citrus fruits used for fresh lemon aromas. Fragrances such as benzyl salicylates, hydroxy citronellal, geraniol, and cinnamic alcohol and essential oils such as ylang-ylang and jasmine are absolute likely culprit allergens for PCD.[5]
Lavender absolute, musk mix, cetyl alcohol, thimerosal sorbic acid germall 11, and benzyl salicylates are the usual components in fairness creams. They are the second most common cause of cosmetic-related PCD.[5] Woo et al.[21] reported patch test positivity in all five patients to henna extract. Cobalt, nickel, and benzyl salicylates are the most common contact allergens. PCD, secondary to lipstick has also been reported with coal tar dyes like CI 15,800 brilliant lake red and 1-phenylazo-2-naphthol derivatives and ester gum, iso-dipalmitoyl diglyceryl sebacate, ricinoleic acid, and dipentaerythritol fatty acid ester. Hayakawa et al. reported slate brown pigmentation secondary to cheek rouge containing musk moskene.[22]
Lately, drug-induced PCD has also been reported. Inui et al.[23] reported 11 (5.91%) out of 186 with hyperpigmentation secondary to diphenyl-cyclopropene in alopecia areata. In another study, García-Gavín et al.[24] reported unusual paradoxical hyperpigmentation secondary to kojic acid lightening agents for lentigines on arms and a patch test positively to kojic acid 1% aqua. There was an unprecedented increase in the incidence of facial pigmentary disease in the recent COVID-19 pandemic. Kim and Lee[25] reported increased incidences of RM in vulnerable individuals exposed to mask-related allergens, leading to contact dermatitis during the COVID-19 outbreak. It has been seen that direct contact with several allergens is typically the cause. However, a few cases have been reported due to exposure to airborne allergens. As facial melanosis is present over photo-exposed areas and some of the causative agents are known to be photosensitizers, UV exposure may be a factor in some cases.[1,11]
ROLE OF AUTOIMMUNITY
Japanese women with Sjogren’s syndrome having anti– Sjogren’s-syndrome-related antigen A [anti-SSA (RO)] positivity present with RM-like lesions involving facial areas secondary to sun exposure. It has been postulated to ultraviolet radiation triggering the expression of the SSA antigen on keratinocytes, which are the targets of circulating anti-SSA antibodies, and results in interface dermatitis and pigment incontinence. However, the pigmentation usually disappears with the implementation of UV protection.[11] Recently, Lai et al.[26] have reported RM-like lesions with other autoimmune diseases, which include lupus erythematosus and thyroiditis.
CLINICAL FEATURES AND EVALUATION
Pigmentation changes in PCD typically appear gradually and persist for a longer time due to the slow pigment clearance.[12] It presents as reticulated slate grayish or brownish hyperpigmentation with slight evidence of preceding erythema, edema or pruritus, that points toward contact dermatitis.[5] Commonly affected areas include the cheeks, ears (external, helix, and lobule), temporal regions, preauricular and nasopharyngeal areas, as well as the upper back and arms. Black, purple, or blue-black color variations are commonly observed.[6] The clinical improvement assessment is mainly done using serial clinical photography, melanin index, and erythema index using spectrophotometry. Some studies have used histopathology as an assessment tool, but it can be cumbersome for patients.[27] Kumarasinghe et al.[3] reported a global consensus that gave specific clinical features and defined it as “numerous fine (few millimeters in size) or reticulate, acquired macules of pigmentation of uncertain etiology occurring on face, neck, and upper chest.” Ashy dermatosis and Lichen Planus Pigmentosus (LPP) are two other conditions whose clinical manifestations may be similar to PCD.[27] Differentiating points have been tabulated below [Table 2].[27] Vinay et al.[28] did an objective scoring to grade the disease severity of ADMH involving the head and neck region. The assessment was done using dermoscopy, and a multiplication was used. Hence, a novel scale commonly known as acquired dermal macular hyperpigmentation area and severity index (DPASI) can be used to assess the severity and extent of the disease. The face is divided into four regions, and the neck is divided into two. The surface area and grade of involvement can be calculated using the formula below. If the score is higher, it corresponds to the disease severity. A maximum score of 40 can be given.
| Acquired dermal macular hyperpigmentation | |||
|---|---|---|---|
| Features | LPP | PCD | EDP/ashy dermatosis |
| Age predilection | Any | Mid-aged | Any |
| Gender | Females>males | Females>males | Females>males |
| Geographical distribution | Primarily reported from India | Primarily reported from Asian countries, mainly Japan | Primarily reported from Mexico and the Latin world |
| Presentation | Discrete dark brown macules mainly involving face and neck flexors, both photo-exposed or photo-unexposed areas. Perioral involvement is typical of LPP. Usually, they are asymptomatic but sometimes can present as mild erythema and burning. | Diffuse or patchy brown macules with ill-defined borders mainly involving the face (forehead, temples) and having a symmetrical presentation. Mostly associated with itching. | Ash-colored polycyclic macules are mainly present over the trunk and proximal extremities. It may sometimes extend to involve the head-neck region. There is an occasional presence of a typical rim of erythema with an elevated border. Usually, they are asymptomatic but occasionally can present with itching. |
| Possible Causation | Sun exposure, fragrances, henna, hair dyes, mustard oils, and amla oils. | Hair dyes, textiles, occupational allergens, drugs | Exposures to ammonium nitrite, radiological contrast, chlorothalonil, intestinal whipworm, cobalt, HIV. |
| Other associations | Lichen planus, Alopecia areata,Vitiligo, Atopy, Autoimmune thyroiditis, Frontal fibrosing alopecia | - | - |
PCD: Pigmented contact dermatitis, LPP: Lichen planus pigmentosus, EDP: Erythema dyschromicum perstans
Further, another critical factor to consider while treating patients is when to consider patients for systemic therapies as per the severity of the disease. Factors considered for disease assessment included - (i) number of new lesions and (ii) increase in existing lesions or evolution of new lesions over the past six weeks [Table 3].[28] Later, Kumaran et al.[29] assessed the reliability and validity of the DPASI scoring system in 55 patients and recommended that it be a relatively validated tool for quantitative assessment of disease severity in Acquired dermal macular hyperpigmentation (ADMH).
| Stage | Progression | New lesions | Area increase |
|---|---|---|---|
| Stage 0 | No | No | No |
| Stage 1 | Very slow | 1–5 | <3 cm2 |
| Stage 2 | Slow | 6–10 | 3–6 cm2 |
| Stage 4 | Rapid | 11–15 | 6–9 cm2 |
FORMULA TO OBTAIN DPASI
DPASI = 2× (%Forehead Involvement × Grade) + 2 × (%Right Cheek Involvement × Grade) + 2 × (%Left cheek involvement × grade) + 1 × (%Central Face Involvement × Grade) + 1.5 × (%Left Neck Involvement × Grade) + 1.5 × (%Right Neck Involvement × Grade)
Dermoscopy is a non-invasive method that can help in PCD diagnosis. Main dermoscopic features include slight scales, pseudo-network, gray dots/globules, follicular keratotic plugs, perifollicular whitish halo, owl’s eye sign telangiectatic vessels, and hypopigmented network patterns.[1,30] Vinay et al.[28] described severity score criteria that included grades I, II, III, and IV based on variables such as dots, globules, and blotches density and pattern. (I). Light brown macules with a sparse distribution of random dots (II). Dots>globules with broken lines and Chinese lettering. (III). Reticulated dots and globules with few blotches (IV). Widespread brownish to blackish color with thick spots, globules, and blotches, and destroyed pigment network.[11] Histopathology shows interface dermatitis as the most common feature encountered. The timing at which the lesion has been biopsied is crucial in the histopathological outcome. Early biopsy shows features of pigmentary incontinence, perivascular or band-like dermal lymphohistiocytic infiltrate, and vacuolar basal layer degeneration, and late biopsy shows features of predominant upper dermal melanophage infiltration.[31] Immunohistochemical analysis shows a more significant proportion of CD4+ cells than CD8+ cells at the patch test positive site; however, it showed equal or slight CD8+ cell predominance in chronic skin lesions.[32]
Patch testing plays an immense role whenever PCD is diagnosed, primarily due to contact allergens. A standard cosmetic or fragrance series containing local allergens is used. Readings are taken at 48 h and 7 days. All patients must undergo closed patch testing on diagnoses of PCD. Photo-patch testing must be done if photoallergen is suspected. Papules, vesicles, or brown pigment may appear at patch test sites and can be interpreted as per the International Contact Dermatitis Research Group (ICDRG) rating system. Repeated open application tests are required if closed patch testing is negative or equivocal, presumably due to reduced antigen concentrations in the allergy and fragrance series.[5,19]
RECENT DIAGNOSTIC ADVANCES
Lu and Jiang[33] used reflectance confocal microscopy (RCM), also known as confocal laser scanning microscopy or skin computer tomography, for evaluating various skin conditions by three-dimensional reconstructions of skin structures by a combination of multiple images obtained at different skin depths that are highly correlating with histopathology and having added advantage of preservation of skin structures. Features favoring RM on RCM are tabulated under [Table 4].[33] Shen et al.[34] found promising results using multimodality skin imaging systems combining optical coherence tomography and new skin diagnosis systems. RM suggestive features included hyper-reflective supranuclear capping, dermal hyper-reflective melanophages, doughnut-shaped spots, blurred dermoepidermal junction suggestive of degenerative basal keratinocytes, and irregular acanthosis and dilated vessels representative of dermal inflammation.
| Histopathological feature | RCM |
|---|---|
| Epidermal inflammatory cell infiltrates | Round to polygonal bright structures |
| Basal layer vacuolization and degeneration | Obscured papillary rims and obliteration of the high refractive ring-like structures around dermal papillae |
| Pigment incontinence | Brightly refractile, plump, oval to stellate-shaped cells and monocyte infiltrates in the superficial dermis. |
| Dilated vessels | Prominent round or linear canalicular structures |
| Perivascular inflammatory cell infiltrate | Mildly refractive and round to polygonal cells around dermal vessels |
| Dilated infundibulum | Black round or oval lumina |
| Hyperkeratotic adnexal infundibula | Highly refractive material within infundibula |
RCM: Reflectance confocal microscopy, RM: Riehl's melanosis
PSYCHOLOGICAL IMPACT
Devastating psychological effects can result from PCD-related deformity on social acceptance, mental health, and self-esteem. Patients with PCD have a significantly higher mean score on the dermatology life quality index (DLQI) and the Melasma Quality of Life Scale (MELASQOL) than those with melasma and healthy controls. Dabas et al.[35] found a strong link between ADMH severity and anxiety and depression, reporting that 18%, 24.1%, and 14.3% of 100 patients with ADMH, respectively, suffered from these conditions. Yadav et al.[36] recently assessed the quality of life in 52 patients with acquired dermal melanosis, including PCD. DLQI was used and showed a moderate effect on 42% of patients.
PATIENT EDUCATION AND RECENT ADVANCES IN TREATMENT
Patients must get proper counseling regarding the causal role of cosmetics or textiles and the likelihood of persistent hyperpigmentation even after discontinuing causative agents. Avoiding the allergen that causes PCD, wearing a broad-spectrum sunscreen, and engaging in sun-protective behavior are general methods for treating the condition.[1,27] Treatment can be broadly classified into three categories [Table 5].[37-46]
| Topical | Oral | Lasers |
|---|---|---|
| Cosmetic camouflage | Oral glycyrrhizin compound | Q-switched Nd-YAG 1064 nm. |
| Hydroquinone | Vitamin C | Intense pulse light systems |
| Corticosteroids | Oral tranexamic acid | Picosecond alexandrite laser |
| Retinoids | Mycophenolate mofetil | Non-ablative fractional thulium fiber laser |
| Vitamin C | ||
| Azelaic acid | ||
| Chemical peels (trichloroacetic acid, glycolic acid and salicylic acid peels) | ||
PCD: Pigmented contact dermatitis
LITERATURE SEARCH
A thorough literature search was done in the PubMed database with the following terms: “Pigmented contact dermatitis” and “treatment,” “Riehl’s melanosis” and “treatment,” and “acquired dermal macular hyperpigmentation” and “treatment” on October 12, 2023. Inclusion criteria included - (i) studies having a sample size of more than three, (ii) English-only articles, (iii) articles after the year 2010, and (iv) all article types except case reports or case series having a sample size of less than three. The search yielded 37 articles, of which only 11 met the inclusion criteria. Different studies have tried various treatment combinations [Table 6].
| S. No. | Study author | Year | Study design | Sample size | Sex (M/F) | Age | Skin type | Sites involved | Treatment given | Treatment interval/Duration of treatment | Follow up | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Li et al.[41] | 2011 | Case Series | n=6 | 1/5 | 27–54 | (Fitzpatrick skin type IV) | Face | Intense pulse light systems with 590, 640, and 695 nm cut-off filters with fluences ranging from 11–17 j/cm2 were used in serial treatments. A triple pulse mode with a pulse width of 3–4 ms and a delay time of 35–40 ms was used with slight erythema as an endpoint. | - | 6-month | Good improvement: 5 Excellent improvement: 1 |
Mild to moderate tolerable pain. Transient erythema and slight edema. Postinflamatory Hyperpigmentation (PIH) |
| 2. | Chung et al.[43] | 2014 | Pilot Study | n=6 | 0/6 | 29–58 | (Koreans, Fitzpatrick III and IV) |
Neck, chin, perioral | Q-switchedNd-YAG laser in dual pulse mode with a fluence, frequency, and spot size of 2–4 J/cm2, 10 Hz and 7 mm, respectively. | 2 weeks | 20 weeks | 50–75% improvement: 4 25–50% improvement: 2. |
No adverse effects |
| 3. | Kwon et al.[45] | 2016 | Prospective Pilot Study | n=8 | 0/8 | 35–61 | (Koreans, Fitzpatrick skin type III–V) |
Face, neck | Low fluence Q switched 1064 Nd YAG laser combined with 4% HQ cream applied topically every night and oral tranexamic acid 250mg/daily. | 3-weeks | 54 weeks | >75% improvement: 3 50–75% improvement: 5. |
No adverse effects |
| 4. | Xu et al.[38] | 2018 | Prospective Pilot Study | n=10 | 0/10 | 35–50 | (Chinese, Fitzpatrick skin type III–V) |
Face, neck | Oral tranexamic acid 250 mg twice a day with oral glycyrrhizin 150 mg daily. | Oral tranexamic acid for 6 months, oral glycyrrhizin for the first 3 months | 24 weeks | 50–75% Improvement: 7 25–50% improvement: 2<25% Improvement: 1 |
No adverse effects |
| 5. | Choi et al.[44] | 2019 | Retrospective Study | n=10 | 0/10 | 46–81 | (Korean) | Face and neck | 1,064-nm Q-switched Nd: YAG laser with fluence starting from 0.9 J/cm2with a 7-mm-sized spot and frequency of 10 Hz. The fluence of the laser was adjusted up to 2.0 J/cm2. 4% HQ cream was concurrently used. | 3 weeks | - | Grade 4 improvements: 7 Grade 3 improvements: 2. However, one patient failed to reach grade 2 improvement even after ten treatment sessions. | Itching: 7 Erythema: 2 None: 3 |
| 6. | Wang et al.[46] | 2019 | Case Presentation | n=3 | 1/2 | 27–52 | (Chinese, Fitzpatrick III and IV) | Face, neck | Combination therapy of oral Glycyrrhizin 150 mg/daily with vitamin C 100 mg/daily, and salicylic acid 30% peels | 2 weeks. | 24 weeks | Significant improvement: 3. | Mild burning |
| 7. | Cho et al.[42] | 2020 | Retrospective Review | n=21 | 1/20 | 46–75 | (Koreans, Fitzpatrick skin type III–IV) |
Face, neck, upper chest | Six sittings of mid-fluence Q-switched Nd-YAG 1064 nm laser (laser intensity of 3.5–5 J/cm2, 5-mm spot size, and 10 Hz frequency). | 40 days | 34 weeks | >75% improvement: 2 50–75%: 8 25–50%: 6<25%: 2 No improvement: 3, and no patient showed worsening. |
Pruritus: 1 Prolonged erythema: 1 Disappeared within 2 weeks. |
| 8. | Bishnoi et al.[39] | 2021 | Open-Label Pilot Study | n=46 | 6/40 Three discontinued study |
18–52 | (Indian, Fitzpatrick III–V) | Face, neck, extending beyond face and neck | Mycophenolate mofetil 2 g/daily dose. | 24 weeks | 36 weeks | >50% decrease in dermal pigmentation area and severity index: 1>40–50%:10>30–40%: 15 | Leucopenia: 1 Transaminitis and hyperbilirubinemia: 2 |
| 9 | Kim et al.[40] | 2021 | Retrospective Chart and Photographic Review | n= 9 | - | - | Koreans | Face, neck |
Three to seven sessions of Non-Ablative 1927 nm TFL (10-20 mj). | 4 Weeks | - | 76%-100%: 1 51%-75%: 6 26%-50%: 2 DPASI decreased from 9.55 to 5.25 |
Transient erythema |
| 10 | Park et al.[47] | 2022 | - | n=44 | - | - | Koreans | Facial | Five sessions with Pulsed-type Micro-needling RF | 2 weeks | 4-8 Weeks | Most patients report significant clinical improvements with a post-treatment decrease in MI, EI, CD44, and b-FGF. | No adverse effects |
| 11. | Rani et al.[37] | 2022 | Case Series | n=6 | - | - | Indian | Facial | 15 to 16 sessions of 33% glycolic acid and 7% kojic acid | - | - | Visible clinical improvements in all patients | No adverse effects |
HQ: Hydroquinone, PCD: Pigmented contact dermatitis, TFL: Thulium fiber laser, MI: Melanin index, EI: Erythema index, b-FGF: Basic-fibroblast growth factor; DPASI: Dermal macular hyperpigmentation area and severity index.
DRUG THERAPIES (ORAL AND TOPICAL)
Drug therapies, topical or systemic, have not shown promising results when used individually. Several topical agents such as hydroquinone (HQ), azelaic acid, kojic acid, retinoids, glycolic acids, tretinoin, and topical corticosteroids have been tried. Rani et al.[37] studied six PCD patients using a combination topical chemical peel therapy of 33% glycolic acid and 7% kojic acid for 15–16 sessions. Patients were advised to continue their topical demelanizing agents and stringent sun protection. A significant clinical improvement was noted. Oral therapies such as tranexamic acid (TXA), glycyrrhizin oral compound, and mycophenolate mofetil have been used to treat RM with promising results. TXA is a synthetic derivative of lysine. It involves the blocking of interactions between keratinocytes and melanocytes by having a suppressive action over the epidermal melanocytes tyrosinase and inhibiting the plasminogen-plasmin system.[11] Another study by Xu et al.[38] over three months in resistant cases of Riehl’s melanosis involving ten patients. A combination therapy including 500 mg TXA and 150 mg glycyrrhizin oral compound for the first 3 months, followed by 500 mg TXA as a lone therapy for the next three months. Seventy percent of the patients demonstrated a significant decrease in their melanin index with no significant adverse effects. Furthermore, Bishnoi et al.[39] used mycophenolate mofetil 500 mg twice daily for two weeks followed by 1000 mg twice daily for the subsequent 22 weeks. A 12-week follow-up showed that 46 patients resulted in significant improvement in their pigmentation. Side effects included leucopenia, nausea, diarrhea, and transient transaminitis that resolved on drug discontinuation.
LASERS
Laser use in pigmentary disorders has seen a recent upward trend in recent years. Up to now, research has been documented only in the use of Intense pulse light (IPL), Q-switched Nd: YAG (QSNY) lasers, Picosecond Alexandrite Lasers, and Fractional Thulium Fiber Lasers (TFL) with promising results, and with minimal side effects. Kim et al.[40] reported using TFL (10–20 mj) with 3–7 sessions at monthly intervals. A significant dip was seen in the dermal pigmentation score and severity index from 9.55 to 5.25. Side effects included transient erythema and swelling.
IPL is used for various hyperpigmented disorders. It has been found to convert light into heat energy, thereby directly targeting epidermal melanin and making epidermal melanosomes move quickly to the upper layers, thereby resulting in depigmentation.[11] Li et al.[41] did a split-face study with IPL involving six patients with RM. Serial treatment with 8–10 sessions of IPL, and with cutoff filters (590, 640 and 695 nm) and fluence (11–17 j/cm2) was done. Six-month follow-ups reported good to excellent improvements in the average value of Melanin index (MI) and Erythema index (EI). Side effects included post-inflammatory hyperpigmentation and mild to moderate tolerable pain with slight transient erythema. It was hypothesized that accelerated keratinocyte damage and upward transfer of melanosomes with necrotic keratinocyte breakdown of pigment deposits in the dermis and faster melanophage transport were responsible for pigment clearance.
QSNY lasers have shown promising results in pigmentary disorders by induction of non-ablation of target piments and selective photothermolysis. Longer wavelengths can target deeper pigmentation and obtain significant results. Cho et al.[42] used mid-pulsed 1064 nm (parameters: 3.5–5 j/cm2 energy, 5 mm spot size) involving 3–12 sessions at four weekly intervals. About 76.1% of patients reported having moderate improvement using standardized photographs, melasma area and severity index scores, and skin biopsy. Side effects included tolerable itching and prolonged erythema. Another study by Chung et al.[43] reported a case series involving patients with RM who were effectively treated with novel QSNY laser (parameters: dual pulse mode with fluence, frequency, and the spot size of 2–4 J/cm2, 10 Hz, 7 mm, respectively and 140 s interval). Marked and moderate improvements were noted in four and two patients, respectively.
COMBINATION THERAPIES
Combination therapies have been reserved for treatment-resistant cases. It increases the efficacy and reduces the side effects, so better compliance. Choi et al.[44] reported using 1064 nm QSNY lasers (parameters: 2.0 j/cm2) thrice weekly combined with topical 4% HQ cream. Seventy percent of the patients reported near-total improvement with guttate hypopigmentation and transient pigment aggravation as side effects. Another prospective study by Kwon et al.[45] involved eight patients having resistant Riehl’s melanosis. A daily dose of 250 mg of TXA, three weekly QSNY laser treatment (parameters: 1.1–1.3 j/cm2), and four ℅ HQ cream applied every night was given. Three of the eight patients reported an almost clear lesion, while the rest responded with marked improvement. No significant side effects were reported. The best outcome was seen with Wang et al.[46] in which a combination therapy of oral Glycyrrhizin 150 mg/daily, 100 mg of Vitamin C daily, and 30% peels with salicylic acids were used every 2 weeks. At 24 weeks, all three patients had significant improvement, with mild burning reported as a side effect.
Recently, Park et al.[47] reported a novel therapeutic technique using pulsed-type microneedling radiofrequency and evaluated its effectiveness and safety in patients with RM. Most patients report significant clinical improvements with a post-treatment decrease in CD44 and Basic-Fibroblast Growth Factor (b-FGF). No adverse effect was encountered.
PROGNOSIS
Overall, the prognosis of PCD is satisfactory. Due to the melanin’s slow engulfment by melanophages, the pigment usually takes more time in resolution and is therefore resistant to treatment, especially to individual treatment modalities. However, recently, promising results have been found with combination therapies. However, still, to date, no specific treatment modality has been recommended.
Limitation of available literature
There is a lack of randomized control trials
There are no trials on females of lactating age groups
Studies with smaller sample sizes
Lack of standardized laser parameters to be used
Shorter follow-up trials
A standardized treatment outcome measurement tool.
CONCLUSION
PCD is a condition that has been studied and learned about extensively over the years. Our ability to identify PCD mimickers and the offending allergen has improved due to the development of dermoscopy, patch/photo-patch testing, and other diagnostic tools. Although there are still many aspects of PCD that still need to be fully explored, the current understanding of this condition will aid in better management and future research.
Ethical Approval
The Institutional Ethics Committee approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Research advances in the treatment of Riehl's Melanosis. Clin Cosmet Investig Dermatol. 2023;16:1181-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired dermal macular hyperpigmentation: An update. Indian Dermatol Online J. 2021;12:663.
- [CrossRef] [PubMed] [Google Scholar]
- A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl's melanosis. Int J Dermatol. 2019;58:263-72.
- [CrossRef] [PubMed] [Google Scholar]
- A Delphi consensus on the nomenclature and diagnosis of lichen planus pigmentosus and related entities. Indian J Dermatol Venereol Leprol. 2023;89:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Melanodermitis liquenoidea de Hoffmann y Habermann Hoffmann and Habermann melanodermatitis toxica lichenoides. Sem Med. 1951;99:577-82.
- [Google Scholar]
- Contact dermatitis due to an optical whitener in washing powders. Br J Dermatol. 1969;81:799-803.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented cosmetic dermatitis. Int J Dermatol. 1984;23:299-305.
- [CrossRef] [PubMed] [Google Scholar]
- Unveiling the mystery of Riehl's melanosis: An update from pathogenesis, diagnosis to treatment. Pigment Cell Melanoma Res. 2023;36:455-67.
- [CrossRef] [PubMed] [Google Scholar]
- Guanine deaminase in human epidermal keratinocytes contributes to skin pigmentation. Molecules. 2020;25:2637.
- [CrossRef] [PubMed] [Google Scholar]
- CCN1/Cyr61 stimulates melanogenesis through integrin α6β, p38 MAPK, and ERK1/2 signaling pathways in human epidermal melanocytes. J Invest Dermatol. 2018;138:1825-33.
- [CrossRef] [PubMed] [Google Scholar]
- Melanogenic properties and expression profiles of melanogenic paracrine molecules in Riehl's melanosis. Int J Mol Sci. 2020;21:1695.
- [CrossRef] [PubMed] [Google Scholar]
- Paracrine roles of hormone receptors in Riehl's melanosis: A quantitative analysis of oestrogen and progesterone receptor expression patterns. Exp Dermatol. 2021;30:396-401.
- [CrossRef] [PubMed] [Google Scholar]
- Facial melanoses: Indian perspective. Indian J Dermatol Venereol Leprol. 2011;77:552-63.
- [CrossRef] [PubMed] [Google Scholar]
- Kumkum-induced allergic contact dermatitis: Are we missing the actual culprit? Indian J Dermatol Venereol Leprol. 2018;84:153-6.
- [CrossRef] [PubMed] [Google Scholar]
- Contact sensitization to hair colours in acquired dermal macular hyperpigmentation: Results from a patch and photo-patch test study of 108 patients. J Eur Acad Dermatol Venereol. 2019;33:1349-57.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and allergens in pigmented cosmetic dermatitis and allergic contact dermatitis to cosmetics in India. Dermatitis. 2018;29:264-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Rep. 2020;6:476-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired diffuse slate-grey facial dyspigmentation due to henna: An unrecognized cause of pigment contact dermatitis in Korean patients. Eur J Dermatol. 2018;28:644-8.
- [CrossRef] [PubMed] [Google Scholar]
- Airborne pigmented contact dermatitis due to musk ambrette in incense. Contact Dermatitis. 1987;16:96-8.
- [CrossRef] [Google Scholar]
- Pigmented contact dermatitis due to therapeutic sensitizer as complication of contact immunotherapy in alopecia areata. J Dermatol. 2010;37:888-93.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermatitis. 2010;62:63-4.
- [CrossRef] [PubMed] [Google Scholar]
- Riehl's melanosis caused by occupational exposure to personal protective equipment. Contact Dermatitis. 2021;85:720-1.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of Riehl's melanosis, lupus erythematosus and thyroiditis in a patient. Clin Cosmet Investig Dermatol. 2022;15:1809-13.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired dermal macular hyperpigmentation: An overview of the recent updates. Int J Dermatol. 2023;62:1447-57.
- [CrossRef] [PubMed] [Google Scholar]
- A novel scale for measurement of acquired dermal macular hyperpigmentation severity. J Eur Acad Dermatol Venereol. 2018;32:e251-3.
- [CrossRef] [Google Scholar]
- Reliability assessment and validation of the dermal pigmentation area and severity index: A new scoring method for acquired dermal macular hyperpigmentation. J Eur Acad Dermatol Venereol. 2019;33:1386-92.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoscopic evaluation and histopathological correlation of acquired dermal macular hyperpigmentation. Int J Dermatol. 2017;56:1395-9.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological features of Riehl melanosis. Am J Dermatopathol. 2020;42:117-21.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented facial contact dermatitis to benzyl salicylate: A comparative histopathological and immunohistochemical study of the involved skin and the positive patch test site. Am J Dermatopathol. 2019;41:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Progress in the application of reflectance confocal microscopy in dermatology. Postepy Dermatol Alergol. 2021;38:709-15.
- [CrossRef] [PubMed] [Google Scholar]
- Riehl's melanosis: A multimodality, in vivo, real-time skin imaging study with cellular resolution optical coherence tomography and advanced skin diagnosis system in a tertiary medical center. Bioengineering (Basel). 2022;9:419.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34:392-9.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in patients with acquired pigmentation: An observational study. J Cosmet Dermatol. 2018;17:1293-4.
- [CrossRef] [PubMed] [Google Scholar]
- Chemical peel as an adjuvant treatment in pigmented contact dermatitis: A case series. J Cosmet Laser Ther. 2022;24:112-7.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study of oral tranexamic acid and Glycyrrhizin compound in treating recalcitrant Riehl's melanosis. J Cosmet Dermatol. 2019;18:286-92.
- [CrossRef] [PubMed] [Google Scholar]
- Oral mycophenolate mofetil in the treatment of acquired dermal macular hyperpigmentation: An open-label pilot study. Australas J Dermatol. 2021;62:278-85.
- [CrossRef] [PubMed] [Google Scholar]
- Non-ablative 1927 nm fractional thulium fiber laser: New, promising treatment modality for Riehl's melanosis. Lasers Surg Med. 2021;53:640-6.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study of intense pulsed light in the treatment of Riehl's melanosis. Dermatol Surg. 2011;37:119-22.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of Riehl's melanosis with mid-fluence Q-switched Nd: YAG 1064-nm laser. Lasers Surg Med. 2020;52:753-60.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study of a novel dual--pulsed 1064 nm Q-switched Nd: YAG laser to treat Riehl's melanosis. J Cosmet Laser Ther. 2014;16:290-2.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of clinical features and treatment outcomes using 1,064-nm Nd-YAG laser with topical hydroquinone in patients with Riehl's melanosis: A retrospective study in 10 patients. Ann Dermatol. 2019;31:127-32.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study for triple combination therapy with a low-fluence 1064 nm Q-switched Nd: YAG laser, hydroquinone cream and oral tranexamic acid for recalcitrant riehl's melanosis. J Dermatol Treat. 2017;28:155-9.
- [CrossRef] [PubMed] [Google Scholar]
- Combination therapy with salicylic acid chemical peels, glycyrrhizin compound, and vitamin C for Riehl's melanosis. J Cosmet Dermatol. 2020;19:1377-80.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic effects of new pulsed-type microneedling radiofrequency for refractory facial pigmentary disorders. Dermatol Surg. 2022;48:327-33.
- [CrossRef] [PubMed] [Google Scholar]







