Translate this page into:
Heat urticaria - Characterizing the population from an urticaria center of reference and excellence (UCARE)
*Corresponding author: Sérgio Duarte Dortas Junior, Department of Immunology, Hospital Universitário Clementino Fraga Filho (HUCFF-UFRJ), Rio de Janeiro, Brazil. sdortasjr@medicina.ufrj.br
-
Received: ,
Accepted: ,
How to cite this article: Duarte Dortas SD. Jr., Azizi GG, Valle SO. Heat urticaria - characterizing the population from an urticaria center of reference and excellence (UCARE). Indian J Skin Allergy. 2024;3:74-6. doi: 10.25259/IJSA_51_2023
Dear Editor,
Heat urticaria (HU) is a rare type of chronic inducible urticaria (CIndU).[1] HU is characterized by well-demarcated wheals appearing soon after heat exposure. Most cases occur in females (82%), with the average age of onset being 34.4 years, spanning from as early as 4 to as late as 78 years.[2,3]
The subtypes include, localized and generalized, depending on the presence of the reaction around directly exposed skin or involvement of distant areas, respectively.[1,3,4] Wheals appear 2–15 min after exposure and can persist for 1–3 h. Some patients may present with systemic manifestations such as syncope, fatigue, nausea, vomiting, abdominal pain, fever, and dyspnea. This picture occurs, particularly, if the areas affected are extensive.[2]
Diagnosis is confirmed by applying a warm stimulus on the surface skin forearm volar for 5 min with a 10-min observation period.[1-3] TempTest® can be employed, offering the advantage of determining temperature thresholds [Figure 1].[1,2]
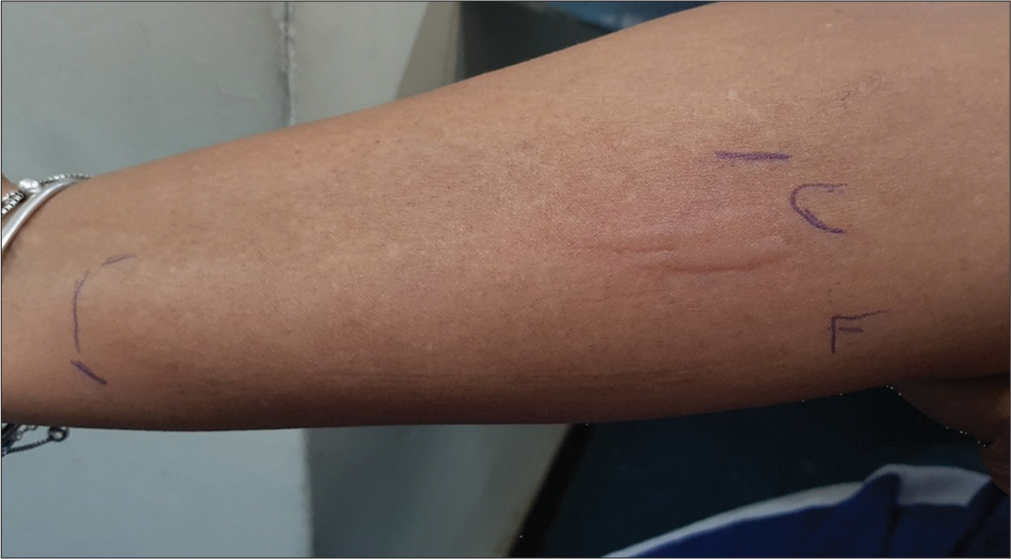
- Heat urticaria positive TempTest®.
HU is often treated with non-sedating antihistamines at recommended doses or even high doses (up to four fold). There are reports of the use of omalizumab (OMA) in refractory cases of patients only with HU or associated with chronic spontaneous urticaria (CSU)with symptom control.[3-5]
A retrospective study was carried out on patients diagnosed with HU and referred to an Urticaria Center of Reference and Excellence (UCARE) (www.ga2len-ucare.com) from 2015 to 2022. Patients were characterized by demographics, comorbidities, CIndU provocation test results, and therapeutic regimens.
The diagnosis of HU was based on a clinical history of the development of hives and/or angioedema after exposure to heat. These diagnoses were confirmed through provocation with TempTest®4.0 [Figure 1]. The subsequent analysis employed descriptive statistics to present categorical variables as frequencies and continuous variables as mean values.
Among the identified patients, six were female. The average age at the time of the study was 44.7 years, with symptoms typically arising around the age of 34. When considering past medical histories, four patients had hypertension, while two others reported hypothyroidism, and one had allergic rhinitis. All patients underwent total immunoglobulin E (IgE) testing, with six exhibiting levels exceeding 40 kU/L and two testing positive for anti-thyroid peroxidase antibodies (anti-TPOs). A striking 6 (86%) of patients additionally grappled with another form of CIndU (dermographism) and CSU.
Patients underwent TempTest®4.0 with mean Heat Temperature Threshold (HTT) 39°C (37–43°C). Significantly, in two patients, symptoms had manifested before their 18th year. Sample characteristics are summarized in Table 1. Pharmacological treatment was prescribed to all patients. One patient was administered recommended doses of antihistamines, while a staggering 6 (86%) of patients were prescribed antihistamines up to four times daily In instances where symptoms remained refractory to antihistamines, three patients underwent additional treatment with OMA, ultimately achieving disease control.
| Patients | 07 (100%) |
| Sex | |
| Female | 06 (86%) |
| Male | 01 (14%) |
| Age, mean (y) | 44,7 (14–60) |
| Association CSU | 06 (86%) |
| Association with other CIndU | 06 patients with dermographism (86%) |
| Autoimmune hypothyroidism (positive IgG anti-TPO) | 02 (29%) |
| Atopic disease | 01 (14%) [Allergic rhinitis] |
| High blood pressure | 04 (57%) |
| Eosinophils mean (cells/mm3) | 521 (475–550) |
| High ESR mean (mm/h) | 17.3 (13–22) |
| Total IgE mean (kU/L) | 57.3 (19–105) |
| Positive temperature in provocation test (TempTest®4–44°C), mean |
39 (37–43) |
CSU: Chronic spontaneous urticaria, CIndU: Chronic inducible urticaria, IgG: Immunoglobulin G, IgE: Immunoglobulin E, Anti-TPO: Anti-thyroid peroxidase, ESR: Erythrocyte sedimentation rate
HU is known as a rare type of CIndU, with around 60 cases described in a recently published comprehensive review.[3] By performing a retrospective review, seven HU patients were identified. As in a previously published data, a predominance of females was found, with the same described mean age of symptoms onset.[3]
Atopy relevance in patients with CIndU is a case of discussion, as there are some reports associating it with the severity or persistence of urticaria.[3] Among our patients, two reported respiratory allergies, but there was no correlation with severity.
Diagnostic workup for HU encompasses a comprehensive evaluation, including a thorough history, physical examination, basic laboratory tests such as a differential blood count, and assessments of C-reactive protein and/or erythrocyte sedimentation rate.[6] In addition, total IgE and anti-TPO are integral components.[6,7] The presence of autoallergic CSU, characterized by IgE autoantibodies, was noted in 6 (86%) of our patients, denoted by total IgE levels exceeding 40 kU/L.
Six patients presented both CSU and CIndU. Recent studies have shown that CSU with concomitant CIndU is associated with refractory and longer disease.[8]
The average reported threshold temperature is 44°C.[3] In our population, mean HTT was 39°C, ranging from 37°C to 44°C.
Current guidelines advise non-pharmacologic management as patient education about heat avoidance techniques after identifying triggers and thresholds. First-line pharmacologic therapy of choice is non-sedating H1-antihistamines up to a 4-fold dose. The addition of OMA,even in higher doses, as adjunctive therapy to H1-antihistamines is the next step. In recalcitrant cases, cyclosporine should be considered as the third step.[6] Most of our HU patients needed higher doses of antihistamines to control the disease, 2 (29%) with the addition of OMA. These patients presented total IgE levels > 100 kU/L. Limitations of this study are the retrospective character of the study and the small number of patients.
HU is a rare disease with a substantial impairment in patients’ quality of life. To further elucidate the complexities of HU, it becomes evident that prospective, multicentric studies of greater scale are warranted. Such endeavors could provide valuable insights into risk factors and the persistence of HU. Moreover, the approval of OMA and the emergence of new drugs could hold the key to enhanced therapeutic outcomes for patients contending with antihistamine-refractory HU.
Ethical approval
The study was submitted and approved by Hospital Universitário Clementino Fraga Filho Research Ethics Committee, CAAE 88430318.0.0000.5257.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Sergio Dortas Junior declares he received lecture fees from AstraZeneca Brazil, GSK Brazil, and Sanofi Brazil. Solange Valle declares she received lecture fees from CSL Brazil and Takeda Brazil. Sergio Dortas Junior and Solange Valle declare they received lecture fees from Novartis Brazil. Guilherme Azizi has no conflicts of interest to declare.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The definition, diagnostic testing, and management of chronic inducible urticarias-The EAACI/GA(2) LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy. 2016;71:780-2.
- [CrossRef] [PubMed] [Google Scholar]
- Heat urticaria: A revision of published cases with an update on classification and management. Br J Dermatol. 2016;175:473-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of omalizumab in chronic spontaneous urticaria associated with chronic inducible urticaria. Ann Allergy Asthma Immunol. 2020;125:486-7.
- [CrossRef] [PubMed] [Google Scholar]
- Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J Allergy Clin Immunol. 2018;141:638-49.
- [CrossRef] [PubMed] [Google Scholar]
- The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734-66.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic spontaneous urticaria: The role and relevance of autoreactivity, autoimmunity, and autoallergy. J Allergy Clin Immunol Pract. 2023;11:2302-8.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226-36.
- [CrossRef] [PubMed] [Google Scholar]





