Translate this page into:
Genital contact dermatitis
*Corresponding author: Rashmi Jindal, Department of Dermatology, Himalayan Institute of Medical Sciences, SRHU, Dehradun, Uttarakhand, India. rashmijindal98@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jindal R. Genital contact dermatitis. Indian J Skin Allergy. 2025;4:43-51. doi: 10.25259/IJSA_56_2024
Abstract
The genital region is uniquely susceptible to both irritant and allergic contact dermatitis (ACD) due to its delicate anatomy, moist environment, and frequent exposure to potential irritants and allergens. Factors such as friction, maceration, and overuse of hygiene products significantly compromise the skin barrier, increasing the risk of dermatitis. The region’s sensitivity is further exacerbated by physiological changes, such as reduced estrogen levels in postmenopausal women, which heighten susceptibility to external agents. A detailed clinical history plays a critical role in diagnosing genital contact dermatitis (CD). Key elements include symptom onset, triggers, hygiene habits, and exposure to products used personally or by sexual partners. This thorough exploration often identifies potential irritants and allergens overlooked in a routine examination. Irritant CD (ICD) in the genital area typically presents as burning, stinging, and erythema soon after exposure to irritants. Chronic ICD may lead to scaling and lichenification. In contrast, ACD arises from delayed hypersensitivity to allergens, presenting as pruritus, erythema, and, in severe cases, vesiculobullous eruptions. Common agents implicated in ICD include soaps, urine, sweat, and certain hygiene sprays, while ACD is often triggered by allergens such as fragrances, topical medications, preservatives, and rubber components. Patch testing is a cornerstone of diagnosing genital ACD. It identifies specific allergens responsible for the dermatitis and helps in differentiating between relevant and incidental reactions. Expanding patch test series to include additional potential allergens, such as personal care products or items used by partners, enhances diagnostic accuracy. The repeat open application test is another valuable tool, particularly when patch testing yields inconclusive results. Management of genital CD primarily involves strict avoidance of identified irritants and allergens. Patients should cease using unnecessary topical medications and adopt hypoallergenic alternatives. Education on proper genital care, including the use of fragrance-free and dye-free products, is essential. Topical corticosteroids, calcineurin inhibitors, or phosphodiesterase-4 inhibitors may be prescribed for short-term relief.
Keywords
Allergic contact dermatitis
Fragrances
Genital
Irritant contact dermatitis
Medicaments
Preservatives
INTRODUCTION
The genital region’s unique anatomical and physiological characteristics make it distinct from other body parts. Its moist mucosa, complex surface architecture, and frequent exposure to friction and body secretions pose difficulty in the diagnosis of dermatological conditions. Social and cultural stigmas associated with genital diseases often lead to delayed presentation, complicating diagnosis and treatment. Furthermore, the region’s normal appearance varies widely, influenced by age, hormonal factors, and skin tone, which can obscure pathological changes.[1] The overlapping presentations of primary dermatoses and secondary infections add to the diagnostic complexity. Dermatologists face numerous challenges in this area, including difficulty in examination due to patient embarrassment, subtle signs of disease, and the frequent involvement of multiple medical specialties. Understanding these unique aspects is crucial for accurate diagnosis and effective management. Genital contact dermatitis (CD) includes vulval CD, perianal CD, and scrotal CD. The genital region is at a high-risk site for contact sensitization as its delicate skin barrier is frequently compromised by friction, maceration, and exposure to irritants from body secretions and hygiene products. Overzealous cleaning practices, often driven by cultural norms, further degrade the barrier function.[2] In postmenopausal women, low estrogen levels exacerbate vulnerability.[3,4] Vulvar tissue’s increased permeability compared to other body regions due to differences in hydration, occlusion, and susceptibility to friction further heightens this risk. Studies highlight the high prevalence of contact sensitization in this area, underscoring the need for heightened awareness and preventive measures.
IMPORTANCE OF A GOOD HISTORY AND PHYSICAL EXAMINATION
A detailed history and thorough physical examination are pivotal in diagnosing genital CD. Key historical elements include symptom chronology, triggers, personal or family history of atopy, personal and partner product use (to identify connubial or consort Allergic CD), and routine care regimens. Sexual and medical history provides context for possible exposures. Meticulous analysis of personal practices is essential for identifying potential irritants and allergens. Specific areas to address include hygiene habits (e.g., use of panty liners, baby wipes, or feminine products), clothing choices, and application of topical agents such as lubricants, spermicides, or deodorant soaps. Over 60% of patients in a vulvar disease study described adverse hygiene or self-treatment practices when specifically questioned, emphasizing the need for detailed inquiry.[5]
Physical examination should encompass the entire anogenital area and adjacent skin, noting erythema, lichenification, pigmentation changes, and erosions. Observing for signs of systemic skin diseases and performing a speculum examination, when necessary, ensures a comprehensive assessment. Visual inspection can identify signs of inflammation, such as erythema, fissures, and scaling, as well as changes in vulvar architecture.
TYPES OF CD
CD in the genital region is broadly categorized into irritant CD (ICD) and allergic CD (ACD). Each has distinct etiologies, clinical presentations, and management strategies. In the genital area, differentiating between ICD and ACD can be challenging due to overlapping symptoms. However, soft pointers can aid in diagnosis. ICD often results from a direct toxic effect of irritants, such as soaps or urine, and is marked by burning and irritation that can appear within minutes to hours of exposure. In contrast, ACD stems from a delayed-type hypersensitivity reaction to allergens in sensitized individuals, presenting primarily as itching that typically develops 24–48 h post-exposure.[6] While ICD is common in children wearing diapers, ACD is less frequent in this population.[7] Recognizing these subtle differences, along with the exposure history, can guide appropriate management.
ICD
ICD manifests as burning, stinging, and erythema, evolving rapidly upon exposure to irritants. Chronic exposure may lead to scaling and lichenification. The severity depends on the irritant’s concentration and exposure duration. ICD has three predominant clinical presentations. Acute ICD resembles a chemical burn and occurs following exposure to a potent caustic irritant such as phenol, podophyllin, podofilox, imiquimod, trichloroacetic acid, or solvents [Figure 1]. These substances are often prescribed for primary conditions like genital warts or skin tags, but misuse or improper self-application by patients frequently leads to what is termed iatrogenic ICD. Increased ICD following the application of imiquimod for genital warts has been well reported.[4] In pediatric populations, diaper dermatitis from prolonged exposure to urine and feces is a common form of ICD [Figure 2].[4] Diaper dermatitis can sometimes be caused by ACD, which occurs due to sensitivity to allergens present in the diaper materials. Common allergens include diaper dyes, fragrances, elastic bands, and adhesives. These substances can trigger localized skin reactions, leading to irritation and inflammation in the diaper area. Chronic cumulative ICD develops gradually after repeated exposure to weak irritants. Common culprits include feminine hygiene sprays, deodorants, perfumes, soaps, sweat, and urine. A third presentation, known as sensory irritation, is characterized by stinging and burning sensations without visible skin changes. This form is often overlooked in clinical assessments. Prolonged exposure to irritants causes gradual disruption of the skin’s natural barrier, leading to a heightened inflammatory response.[8] The genital region’s naturally moist environment further aggravates this condition, allowing irritants to persist longer on the skin surface.[6,9,10] Research has also shown that wearing occlusive clothing, such as nylon or synthetic fabrics, exacerbates the risk of ICD by trapping moisture and sweat. Table 1 enlists the common genital irritants.[4,11,12]

- Iatrogenic irritant contact dermatitis.
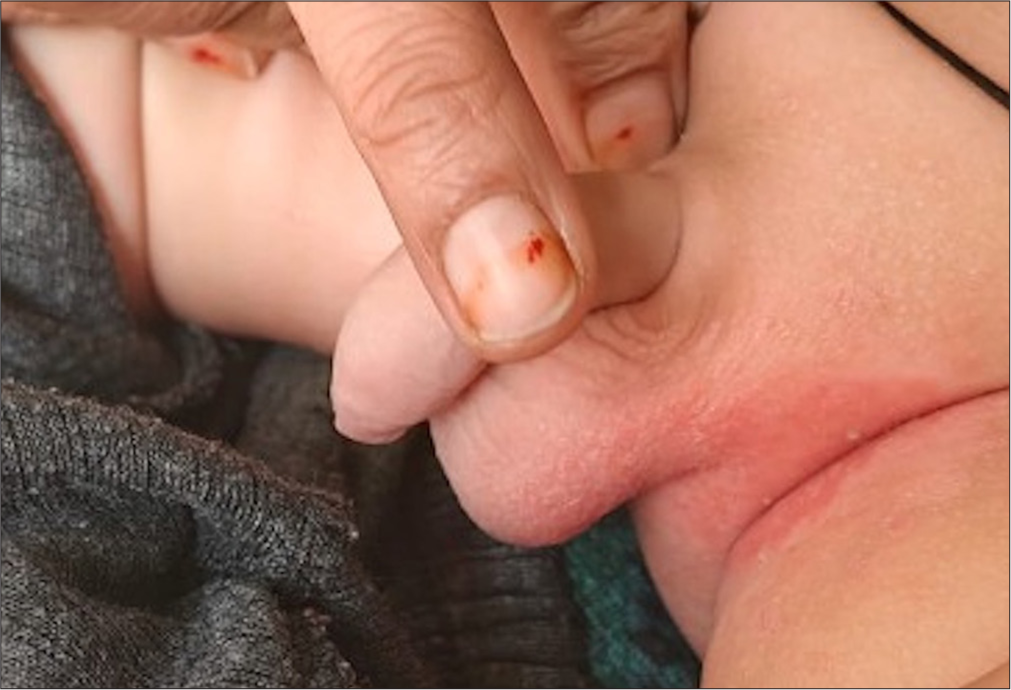
- Diaper dermatitis: A common manifestation of irritant contact dermatitis in children.
| Strong irritants (caustics) | Cumulative irritants | Abrasive contactants |
|---|---|---|
|
|
|
ACD
Genital ACD is an uncommon but distressing condition that affects both men and women. Its impact on quality of life can be profound, causing discomfort, sleep disturbances, and sexual dysfunction.[4,13] Due to cultural stigmas, many patients delay seeking medical attention, often exacerbating their condition by self-treating with over-the-counter (OTC) products that may contain allergens.[11] The prevalence of genital ACD varies significantly. Studies from specialized CD clinics have found that 26–30% of patients with genital dermatitis exhibit relevant positive patch test reactions.[13,14] Notably, no significant gender-based differences in prevalence have been reported. However, factors such as advanced age and certain demographic characteristics, like being white, are associated with a higher risk.[15] Age-related vulnerability is linked to conditions like urinary incontinence and hormonal changes in older women, which compromise the skin barrier, facilitating allergen penetration.[4,16]
Subacute genital ICD can act as a predisposing factor for the development of ACD in the genital area. This is primarily due to cumulative skin barrier damage, which gradually weakens the protective function of the skin.[6] Conditions that compromise skin integrity, such as lichen sclerosus, lichen planus, or lichen simplex chronicus, further exacerbate this dysfunction.[1,4] The compromised skin barrier thus creates an environment that is more susceptible to allergen penetration, sensitization, and the subsequent development of ACD. Patch testing revealed that almost half of the women with vulval issues had at least one relevant positive reaction, with medicaments being the most common allergen.[17] Recognizing and addressing subacute ICD early may help mitigate the risk of progressing to ACD. The impact of atopy on positive patch test reactions in patients with genital ACD has been inconsistently reported in the literature. Some studies suggest that atopics exhibit a slightly higher rate of relevant positive patch test reactions compared to non-atopics.[16] However, other studies indicate that although the overall rate of positive patch test reactions is greater in atopics, the proportion of reactions deemed clinically relevant is comparable between atopics and non-atopics. These findings highlight the need for further research to clarify the role of atopy in influencing patch test outcomes and their clinical significance in genital ACD.[18]
ACD presents with three distinct clinical manifestations. Acute symptoms include painful erythema, vesiculobullous eruptions, and erosions [Figure 3]. In the sub-acute phase, patients typically experience, erythema, and swelling of the genitalia associated with pruritus [Figure 4]. Over time, if the condition becomes chronic, lichenification and hyperpigmentation develop, particularly over areas subjected to recurrent friction [Figure 5].[4] The primary culprits of genital ACD include topical medications, fragrances, preservatives, rubber products, and vehicle components.[3,14,15] A thorough knowledge of the possible contact allergens of the genital region is essential to aid in effective management [Table 2].

- Acute allergic contact dermatitis manifesting as erythema and erosions of the vulva.

- Sub-acute allergic contact dermatitis manifesting as vulval erythema and edema.
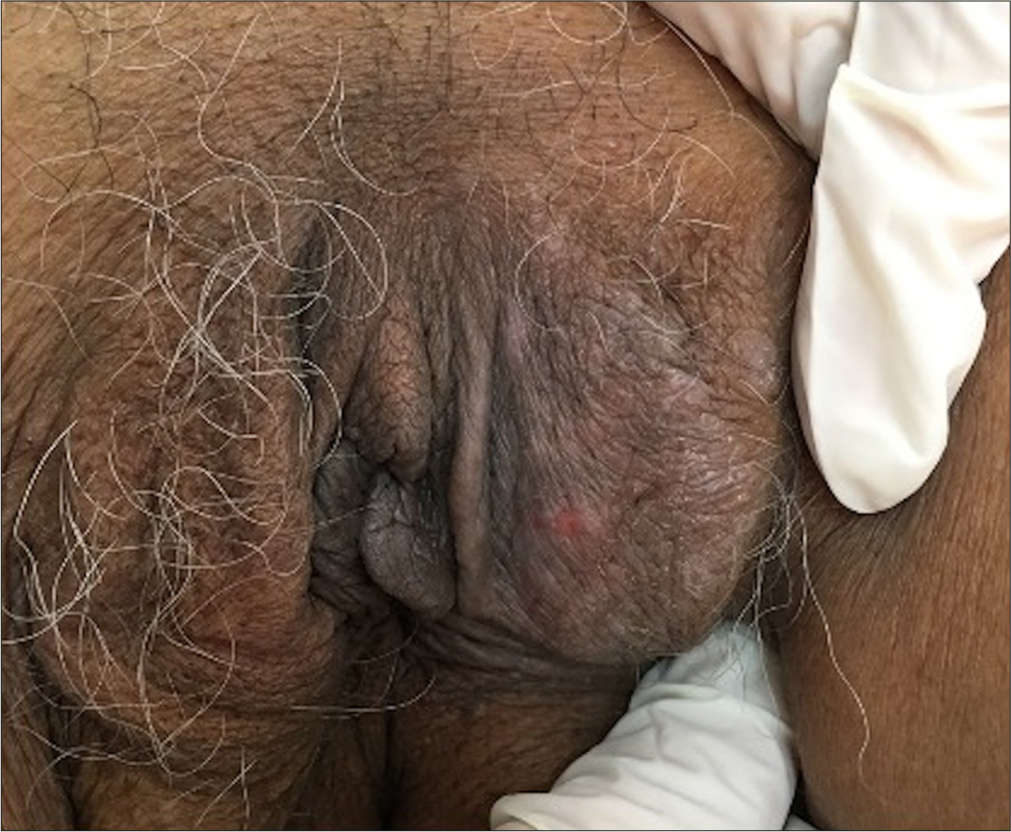
- Chronic allergic contact dermatitis manifesting with lichenification and hyperpigmentation of the vulva.
| Medications | Fragrances | Preservatives | Spices/herbs | Textiles and Resins | Rubber additives/accelerators |
|---|---|---|---|---|---|
| Anesthetics | |||||
|
|
|
|
|
|
| Corticosteroids | |||||
|
|||||
| Antibiotics | |||||
|
|||||
| Antimycotic | |||||
|
|||||
| Antiseptics | |||||
|
|||||
Topical medications
Local anesthetics, antibiotics, and corticosteroids are the most commonly identified allergens resulting in a relevant patch test reaction.[15] This correlates with the knowledge that topical medications are being widely used by patients for co-existing genital dermatoses.[6,9] In a retrospective record-based analysis from a contact allergy unit in Belgium, among 473 patients with perianal/genital lesions, 44 (9%) had positive patch test results to topical drugs or their components, sometimes linked to intimate hygiene cosmetics. The most common allergens were local anesthetics and corticosteroids, while wool alcohols and, to a lesser extent, benzoic acid were frequent sensitizers among vehicle and preservative agents.[19]
Local anesthetics are among the most frequent causes of genital ACD.[3] Benzocaine and dibucaine have been reported to elicit positive patch test reactions in 1.8–4.9% of patients diagnosed with anogenital ACD.[3] Lidocaine and tetracaine have lower sensitization rates, each affecting approximately 0.9% of patients.[3] Benzocaine, a para-aminobenzoic acid derivative, can cross-react with other para-aminobenzoic acid compounds, such as sulfa drugs and hair dyes. Thus a patient with a history of hair dye allergy or sulfa drug allergy presenting with genital pruritus should be suspected for benzocaine-induced CD.[4,20] Benzocaine is included in many OTC medications used for vaginal pruritus, anti-hemorrhoidal creams, and condoms with desensitizing creams to prevent premature ejaculation.[4,18,20] Once an allergy to topical anesthetics develops, they can create a self-perpetuating cycle of temporary symptom relief followed by a worsening of symptoms.[21]
Topical corticosteroids frequently cause sensitization due to their prolonged use in treating pre-existing genital conditions. Triamcinolone and tixocortol are the most common corticosteroid allergens, with sensitization rates of 1.1–3.3%.[9,13] Hydrocortisone, budesonide, and clobetasol exhibit lower rates. Corticosteroid-induced ACD should be suspected when dermatitis worsens with continued steroid application.[15]
Antibiotics and antifungals have also been implicated frequently in genital ACD. Neomycin is a leading antibiotic allergen, with patch test positivity rates between 1.1% and 6.1% in genital ACD cases. Other antibiotics, including framycetin and clindamycin, show sensitization rates of 1–3.1%.[13,16] Antifungal medications, although less commonly included in patch tests, are significant allergens in genital ACD. Terconazole has the highest sensitization rate among antifungals, affecting up to 7% of tested patients.[16] Other antifungals, such as miconazole, econazole, and clotrimazole, show sensitization rates of 1–2%. These agents are frequently found in treatments for fungal infections, increasing the likelihood of exposure.[3,9,16] Nonsteroidal anti-inflammatory drugs like bufexamac and antivirals such as acyclovir are rare but noteworthy allergens. Bufexamac has a sensitization rate of 1.1%, and acyclovir reactions are often attributed to its vehicle components rather than the active ingredient itself.[6]
Fragrances
The term “fragrances” encompasses a vast array of thousands of individual chemical compounds. These substances are widely used and can be found in nearly all categories of personal care and hygiene products. They are common in cosmetic creams, lotions, vaginal washes, hygiene sprays, sanitary pads, wipes, and even toilet paper.
Fragrances are often added to these products to enhance their appeal by masking odors or imparting pleasant scents. However, the complexity of these chemical mixtures can pose a challenge, as they may include allergens or irritants that are not always disclosed on product labels. This lack of transparency can make it difficult for individuals with sensitivities to identify and avoid specific triggers. Furthermore, repeated or prolonged exposure to these fragrance compounds may increase the risk of developing allergic reactions, skin irritation, or other sensitivities, particularly in delicate or sensitive areas of the body. The most common relevant patch test reactions are seen with fragrance mix I or balsam of Peru.[13] Other fragrances include tolu balsam and cinnamic alcohol.[14,15]
Preservatives
Many products contain antimicrobial compounds designed to prevent bacterial or fungal growth and prolong shelf life. Common allergens in this category include methylisothiazolinone (MI) and methylchloroisothiazolinone, which are frequently implicated in wet wipes and flushable moist toilet paper allergies.[22] In an internet-based survey, analysis of feminine hygiene wipes showed all tested wipes to have at least one allergen with a mean of 3.53 allergen per product. Notable allergens were fragrances, tocopherol, botanical extracts, oils, and fruit juices.[23] Sensitization to MI has notably increased, rising from 1.94% in 2009 to 6.02% in 2012.[24] Additionally, preservatives such as formaldehyde and its releasers, including Quaternium-15 and dimethyl-dimethyl hydantoin (DMDM), are associated with clinically relevant genital reactions. These chemicals are prevalent in cosmetic products, lotions, and toiletries, further contributing to sensitization risks.[13,16] Fragrances and preservatives used in condoms and tampons can cause genital ACD. Associated symptoms with tampon ACD include vaginal burning, itching and irritation. Scented tampons can leach out skin sensitizers like a-isomethyl ionone, benzyl salicylate, hexyl cinnamaldehyde, and heliotropine.[25] Patients should be cautioned against trusting labeling claims that a product is “natural,” “fragrance-free,” “soothing,” “organic,” and the like, as products with such labels still contain potential contact allergens. Thiomersal has been implicated commonly as an allergen in toilet seat dermatitis. While it typically does not involve the genital area, its inclusion as a possible cause is relevant when evaluating dermatitis in adjacent regions. It presents as pink-red eczematous lesions on the posterior thighs and is sometimes misdiagnosed as tinea corporis. Patch test positivity has been reported on wood, polypropylene, polyurethane foam, thiomersal, cetrimide, neomycin, cobalt, para-phenylenediamine (PPD), potassium dichromate, and epoxy resin.[26]
Vehicle components
Vehicles in topical medications, often overlooked, can play a significant role in causing skin sensitization and genital ACD. Common components like isopropyl myristate, ethylenediamine, octyldodecanol, propylene glycol, and lanolin/wool alcohol have been identified as potential allergens.[3,6] Prolonged exposure to these substances across various formulations may lead to sensitization.
Wool alcohols and propylene glycol are associated with significant rates of positive patch tests.[3,15] 10.3% of patients with genital ACD have shown clinically relevant reactions to propylene glycol, comparable to allergens like corticosteroids and local anesthetics.[15] However, differentiating true allergic reactions from irritant effects caused by propylene glycol and wool alcohols can be challenging as both are known irritants.
Textiles
Textile components, particularly dyes, and resins, are notable sensitizers in genital ACD. A study found that 12.9% of patients with suspected ACD had reactions to textile components, with dyes like disperse blue (DB) 124, DB 106, and DB 85 being the most common allergens.[27] Genital involvement is common due to friction between the skin and clothing materials. Additionally, lymphomatoid CD, a rare form of ACD associated with textile dyes, may mimic or precede cutaneous lymphoma, requiring careful monitoring.[28]
Spices and herbs
Spices such as coriander, nutmeg, primin, peppermint oil, onion powder, and chamomile are uncommon allergens, but their sensitization rates can reach 13% in certain populations.[29] These allergens are often introduced via hand-to-genital transfer or topical use of herbal remedies. Since ancient times various herbal and home remedies have been used for the treatment of sexually transmitted diseases involving external genitalia. The excretion of these compounds in urine and feces has also been postulated.[29]
Rubber products
Rubber accelerators and anti-oxidants used in rubber processing can cause ACD. Rubber accelerators like tetramethylthiuram disulfide and mercaptobenzothiazole are common allergens in items like condoms and elastic materials and can cause genital ACD in both men and women.[30,31] Condom allergy can however be due to other allergens also such as color, fragrance, flavor, and local anesthetics. Condom catheters worn for prolonged periods can result in ACD involving the penile shaft. Anti-oxidants causing ACD are hydroquinones, phenyl-a-naphthylamine, and propyl PPD.[32]
Resins and adhesives
Resins and adhesives are significant allergen sources in sanitary pads and related products. Among these, colophony (rosin) is commonly implicated and is used in manufacturing various items such as napkins, markers, mascara, and sanitary pads.[33] This resin can cause sensitization through direct skin contact, leading to allergic reactions. Other resins, including acrylates used in adhesives, also pose risks. Sensitization to acrylates is often linked to their presence in absorbent layers of sanitary pads, and even trace amounts of unpolymerized monomers can be highly allergenic.[34] Patients with allergies to these substances may experience recurring dermatitis corresponding with their menstrual cycles, particularly in areas of prolonged exposure [Figure 6]. In some cases, switching to products made from pure cotton or free from adhesives and synthetic resins can significantly reduce symptoms. The growing prevalence of these allergens highlights the need for hypoallergenic alternatives and better labeling to aid sensitive individuals in avoiding exposure.
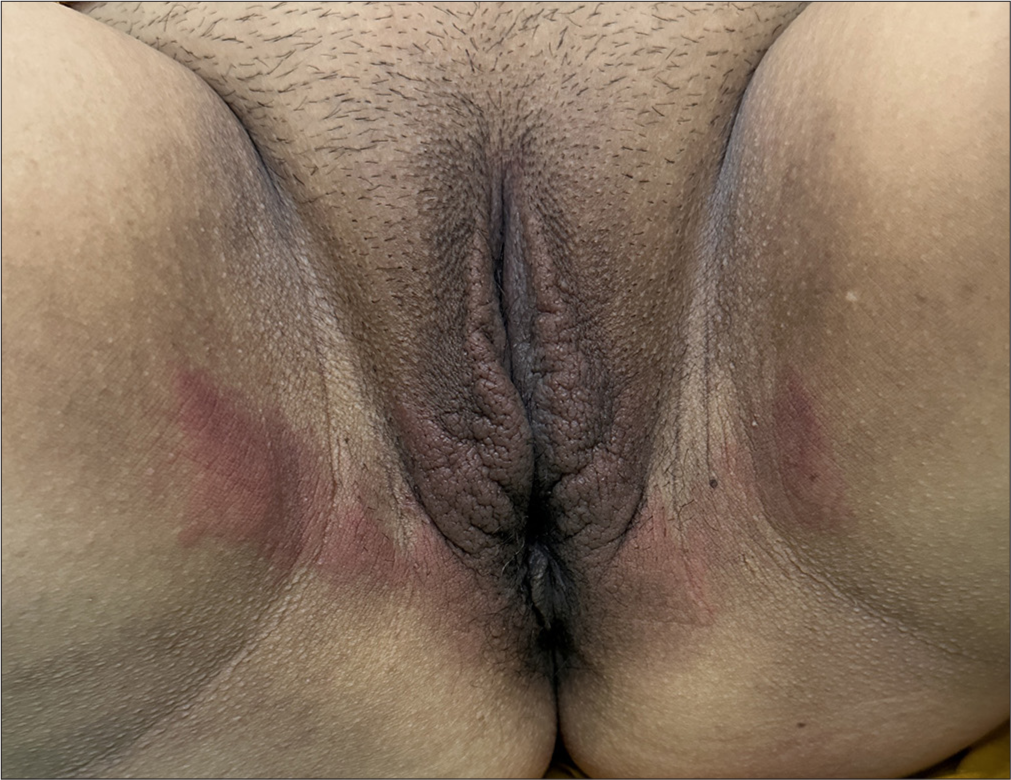
- Allergic contact dermatitis due to sanitary pads.
Systemic CD
Patients sensitized to a topical allergen can develop CD if exposed to that allergen systemically through ingestion, parenteral administration, or inhalation. It can manifest as itching, redness, pain, burning, stinging, and blistering in the acute stage and if left untreated can result in lichenification and hyperpigmentation. This is a less commonly described cause of anogenital dermatitis. The primary groups of allergens implicated include metals such as nickel, cobalt, and chromium; medications like hydroxyzine; plant and herbal products including fragrances, and flavoring agents such as citrus, wine, cinnamon, curry, and vanilla; and preservatives like formaldehyde.[29,35,36] Certain foods like oats, soy products, chocolate, legumes, kale, spinach, and nuts are known to have high nickel content, potentially triggering reactions in sensitive individuals. Propylene glycol, a common allergen known to cause ACD and ICD, can also lead to systemic dermatitis following oral or intravenous exposure through foods and medications. Its widespread use as a thickening or moistening agent in processed foods, such as salad dressings, baked goods, and fast foods, makes complete avoidance challenging. Additionally, certain antihistamines containing propylene glycol have been reported to trigger systemic CD in sensitized people.[37]
DIAGNOSIS AND TESTING
Diagnosis relies on detailed patient history and patch testing. In cases of persistent symptoms, identifying allergen triggers is essential and is best achieved through patch testing with a wide range of known allergens. Indian standard series includes many potential allergens that can help diagnose genital ACD, yet it is not inclusive of all known allergens, and it’s essential to use an extended series. A systematic review of allergic and ICD of vulva analyzing studies published till 2021, identified the common allergens being metals, topical drugs, preservatives, fragrances, and rubber components. The review highlighted the importance of establishing the relevance of a positive patch test as all positive reactions were not relevant. The review further underscores the importance of testing with additional series and patients’ products to avoid missing relevant allergens.[38] The North American 80 Comprehensive Series identifies 52% of allergens commonly associated with genital ACD, including anesthetics, preservatives, fragrances, and topical vehicles.[4] However, it excludes certain allergens, such as some topical medications, rubber additives, and spices. Expanding testing with additional series—covering cosmetic, textile, drug reaction, and bakery-related allergens—can increase coverage to 85%. For untested items like specific spices or uncommon corticosteroids, individualized testing may be necessary.
A thorough history of the patient’s product use is critical in guiding patch-testing choices. Patients should bring in personal products, including cosmetics, OTC items, alternative medicine preparations, and even products used by sexual partners. Commonly implicated items include sanitary pads, hygiene wipes, lubricants, cosmetics, cleansers, and undergarment materials.
The repeat open application test (ROAT) is another diagnostic tool used to identify allergens in suspected cases of genital CD. It involves the repeated application of a suspected allergen, such as a personal care product or medicament, to a specific area of healthy skin (typically the forearm or upper arm) over several days. If an allergic reaction develops at the application site, it confirms the substance as a potential allergen. ROAT is particularly useful in genital CD when patch testing is inconclusive or when the suspected allergen is a product frequently used in the genital area. By mimicking real-world exposure, ROAT helps establish the clinical relevance of a suspected allergen, aiding in accurate diagnosis and management.
MANAGEMENT AND TREATMENT
The cornerstone of treatment is allergen avoidance. Key steps include:
Cessation of topical products: Discontinuing all unnecessary topical medications and over-the-counter products.
Use of hypoallergenic alternatives: Switching to products free of fragrances, dyes, and preservatives. Hypoallergenic soaps, detergents, and sanitary products are recommended.
Topical therapies: Topical corticosteroids can be used for short duration. Nonsteroidal options like calcineurin inhibitors and phosphodiesterase 4 inhibitors may be beneficial for recalcitrant cases.[39]
In severe cases, a short course of oral steroids is beneficial along with antihistamines to control pruritus. Chronic and relapsing cases may require steroid-sparing immunosuppressive agents such as cyclosporine, methotrexate, and azathioprine.
Education and Partner Involvement: Educating patients about safe product use and considering the potential transfer of allergens from partners [Table 3].
| Action | Details |
|---|---|
| Discontinue topical medications | Avoid all over-the-counter medicaments, including antibacterial, antifungal, and irritation creams. Consult a dermatologist before resuming. |
| Avoid hygiene products | Stop using hygiene wipes, sprays, or any similar products on the anogenital area. |
| Cease home remedies | Do not use home remedies containing natural herbs, spices, or oils. |
| Switch moisturizers | Prefer pure petroleum jelly as a moisturizer |
| Use fragrance-free products | Choose fragrance-free soaps, detergents, and sanitary products. Avoid using soap directly on the genital area. |
| Select allergen-free sanitary products | Use pads or tampons free of fragrances, dyes, resins, colophony, and acrylates, preferably made of cotton. |
| Wear appropriate clothing | Choose 100% cotton undergarments. Avoid elastic, nylon, dyed fabrics, and tight-fitting clothing. |
| Choose hypoallergenic condoms | Use condoms made of materials like polyurethane, polyisoprene, or lamb skin that are free of spermicidal lubricants. |
| Use hypoallergenic lubricants | Select water-based personal lubricants that are free from fragrances, glycerine, and parabens. |
CONCLUSION
Genital CD is a multifaceted condition requiring a comprehensive approach to diagnosis and management. Dermatologists must navigate the unique challenges posed by this region’s anatomy, cultural stigmas, and diverse allergen exposures. Thorough history-taking, detailed examination, and tailored treatment plans are essential for improving patient outcomes. Enhanced awareness and preventive strategies can mitigate the burden of this impactful dermatological issue.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Genital contact allergy: A diagnosis missed. Indian J Sex Transm Dis AIDS. 2016;37:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Red plaques with eczematous features In: Lynch PJ, Edwards L, eds. Genital dermatology. Edinburgh: Churchill Livingstone; 1994. p. :38-41.
- [Google Scholar]
- Vulvar dermatoses--irritant and allergic contact dermatitis of the vulva. Dermatology. 2005;210:143-9.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis of the vulva. In Dermatol Ther. 2004;17:20-7.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse behavioral and sexual factors in chronic vulvar disease. Am J Obstet Gynecol. 2000;183:34-8.
- [CrossRef] [PubMed] [Google Scholar]
- Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-4.
- [CrossRef] [PubMed] [Google Scholar]
- Vulvar disease in children: A clinical audit of 130 cases. Pediatr Dermatol. 2000;17:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatologic diseases of the vulva. Clin Dermatol. 1997;15:53-65.
- [CrossRef] [PubMed] [Google Scholar]
- Contact allergic reactions of the vulva: A 14-year review. Dermatitis. 2004;15:131-6.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-43.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis of the vulva. Dermatol Clin. 2010;28:697-706.
- [CrossRef] [PubMed] [Google Scholar]
- Aetiological factors in vulvar dermatitis. J Eur Acad Dermatol Venereol. 2000;14:181-6.
- [CrossRef] [PubMed] [Google Scholar]
- Genital contact dermatitis: A retrospective analysis. Dermatitis. 2010;21:317-20.
- [CrossRef] [PubMed] [Google Scholar]
- Anogenital dermatitis in patients referred for patch testing: Retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 1994-2004. Arch Dermatol. 2008;144:749-55.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72.
- [CrossRef] [PubMed] [Google Scholar]
- Contact sensitivity in pruritus vulvae: Patch test results and clinical outcome. Am J Contact Dermat. 1997;8:137-40.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective study of patch testing in patients with vulval pruritus. Australas J Dermatol. 2008;49:80-5.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-8.
- [CrossRef] [Google Scholar]
- Allergic contact dermatitis to condoms: Description of a clinical case and analytical review of current literature. Immunopharmacol Immunotoxicol. 2004;26:481-5.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis of the vagina and perineum: Causes, incidence of, and differentiating factors. Clin Obstet Gynecol. 2015;58:153-7.
- [CrossRef] [PubMed] [Google Scholar]
- Wet wipe allergens: Retrospective Analysis from the North American contact dermatitis group 2011-2014. Dermatitis. 2017;28:64-9.
- [CrossRef] [PubMed] [Google Scholar]
- A cross-sectional study of contact allergens in feminine hygiene wipes: A possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:e060.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors associated with methylisothiazolinone contact sensitization. Contact Dermatitis. 2013;69:231-8.
- [CrossRef] [Google Scholar]
- Quantitative risk assessment of allergens leaching from menstrual hygiene products. Regul Toxicol Pharmacol. 2022;135:105260.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing in toilet seat dermatitis: A case series of six patients with paediatric atopic dermatitis. Indian Dermatol Online J. 2024;15:616-9.
- [CrossRef] [PubMed] [Google Scholar]
- Textile dermatitis in Israel: A retrospective study. Am J Contact Dermat. 2000;11:26-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphomatoid contact dermatitis associated with textile dye at an unusual location. Indian Dermatol Online J. 2015;6:S24-6.
- [CrossRef] [PubMed] [Google Scholar]
- Anogenital allergic contact dermatitis, the role of spices and flavour allergy. Contact Dermatitis. 2008;59:233-7.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis of the genitals from rubber additives in condoms. Contact Dermatitis. 1993;28:125-6.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic reaction to condom catheter for bladder incontinence. Contact Dermatitis. 2013;69:182-3.
- [CrossRef] [PubMed] [Google Scholar]
- Condom-related allergic contact dermatitis. J Urol. 1995;153:1227-8.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis to a sanitary pad. Australas J Dermatol. 2004;45:234-5.
- [CrossRef] [PubMed] [Google Scholar]
- Acrylate systemic contact dermatitis. Dermatitis. 2015;26:235-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pruritus ani as a manifestation of systemic contact dermatitis: Resolution with dietary nickel restriction. Dermatitis. 2011;22:50-5.
- [CrossRef] [Google Scholar]
- Excipients in oral antihistamines can perpetuate allergic contact dermatitis. Pediatr Dermatol. 2015;32:e242-4.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of allergic and irritant contact dermatitis of the vulva: The most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-62.
- [CrossRef] [PubMed] [Google Scholar]
- Genital allergic contact dermatitis. Dermatitis. 2018;29:112-9.
- [CrossRef] [PubMed] [Google Scholar]






