Translate this page into:
Dasatinib-induced hand foot syndrome followed by bosutinib-induced photoallergic contact dermatitis in a patient of chronic myeloid leukemia: A rare co-occurrence
*Corresponding author: Akash Agarwal, Department of Dermatology, Venereology, Leprosy, Institute of Medical Sciences and SUM Hospital, Bhubaneswar, Odisha, India. akash.22.1995@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pramanik A, Panda M, Panda AK, Agarwal A. Dasatinib-induced hand foot syndrome followed by bosutinib-induced photoallergic contact dermatitis in a patient of chronic myeloid leukemia: A rare co-occurrence. Indian J Skin Allergy. 2024;3:80-2. doi: 10.25259/IJSA_1_2024
Dear Editor,
A 47-year-old man, a known case of chronic myeloid leukemia (CML) for three years in a stable phase, was started on dasatinib as maintenance therapy. Three weeks after initiating the drug, he developed a sudden onset of red elevated lesions on the acral areas which were associated with pain and itching, disrupting his day-to-day activity. Cutaneous examination revealed symmetric, well-demarcated edema, and erythema of both the palms and soles [Figure 1]. There was no history of any recent infections or any variation of the condition with a change in temperature. He could not recall any history of drug reactions in the family members and was reluctant to undergo a biopsy to confirm the diagnosis. Given the temporal correlation of the drug with the cutaneous manifestations, a clinical diagnosis of hand-foot syndrome (HFS; erythrodysesthesia) associated with dasatinib was made. He was advised to discontinue the drug and was started on topical steroids and emollients. Within four weeks of stopping dasatinib, the lesions improved [Figure 2] after which he was started on a different drug of the same family, bosutinib. Following the initiation of bosutinib, he presented with complaints of multiple well-to-ill-defined itchy erythematous and hypopigmented scaly papules and plaques on bilateral forearms and dorsum of hand (predominantly in the photoexposed areas) within 15 days [Figure 3]. The morphology and distribution of the lesions were suggestive of drug-induced photocontact eczema. He had no previous history of photosensitivity or eczema before the drug intake and did not consent for both patch testing and biopsy; hence, a provisional diagnosis of bosutinib- induced photoallergic contact dermatitis was made. He was counseled regarding the causality of the disease and the need for change in the chemotherapeutic agent for the same. He was prescribed a short course of oral corticosteroids, sunscreens, and antihistamines for symptomatic relief. The lesions subsided within three weeks of discontinuation of bosutinib. Due to recurrent adverse effects with the use of tyrosine kinase inhibitors, he was started on azacitidine therapy for the management of underlying chronic myeloid leukemia (CML). He is currently under remission and on regular follow-up.
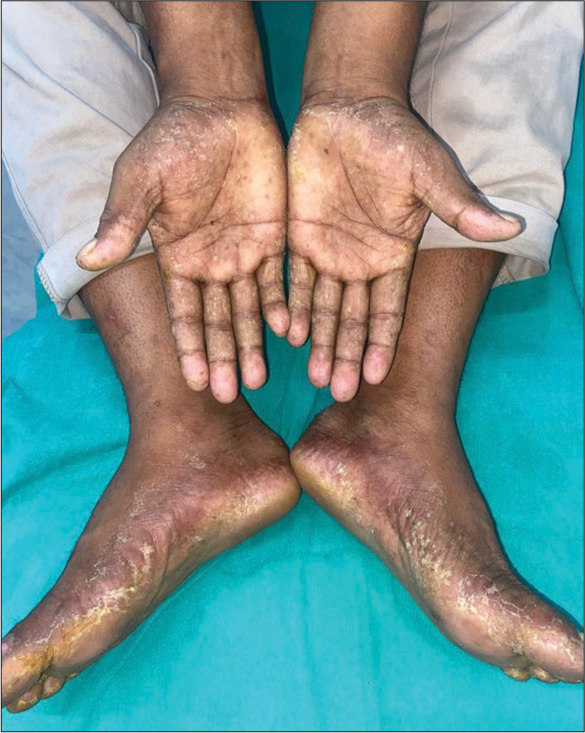
- Painful, symmetric, well-demarcated edema, and erythema on both palms and soles after dasatinib use.

- Resolution of erythema and edema on discontinuing dasatinib.
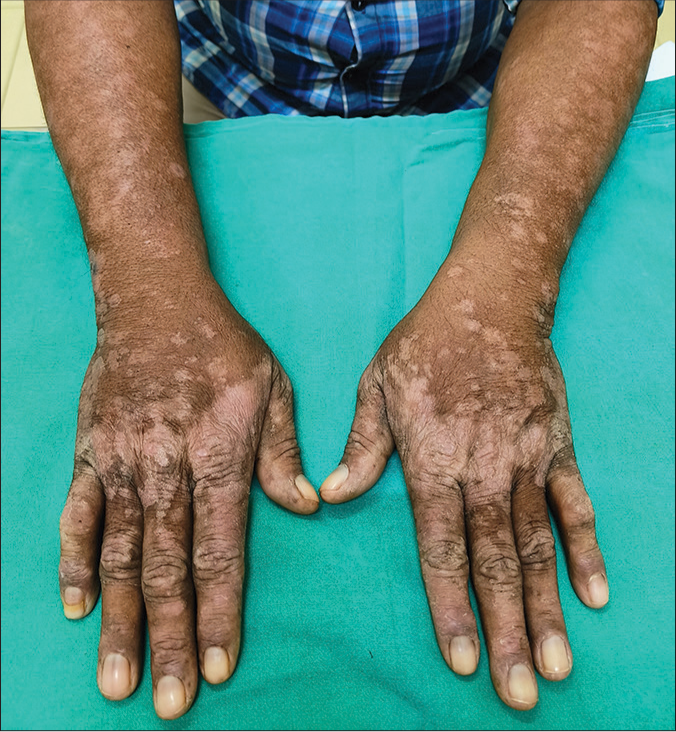
- Multiple well-to-ill-defined itchy erythematous to hypopigmented scaly papules and plaques bilaterally on the forearms and dorsum of hand (predominantly over the photoexposed areas) after starting bosutinib.
Small molecule kinase signal transduction inhibitors, which include dasatinib, and bosutinib, are the first line in the treatment of CML. They act as a potent and selective inhibitors of BCR-ABL fusion protein by competitive inhibition at the ATP-binding site of the enzyme that inhibits tyrosine phosphorylation of the proteins involved in signal transduction. They inhibit intracellular signaling pathways regulating cellular functions, such as proliferation and differentiation in tumor cells. Different molecules target different components of the tyrosine kinase signaling cascade. Their use may be associated with a wide variety of cutaneous side-effects in some patients which might even warrant discontinuation of the drug.[1]
Tyrosine kinase inhibitors such as dasatinib and bosutinib can cause cutaneous adverse effects in the form of erythema, maculopapular eruptions, pruritus, allergic dermatitis, acne, folliculitis,and skin exfoliation.[2,3]
HFS is a commonly documented side effect of chemotherapy drugs. Even though it is not fatal, HFS, without prompt management, can cause serious cutaneous manifestations hampering the quality of life of the patient as well as ongoing chemotherapy. It is usually mild, reversible and resolves with supportive management with short-course steroids, emollients, and antihistamines. Severe cases may require dose reduction or discontinuation of the drug.[4] It has been usually reported with capecitabine, docetaxel, 5-fluorouracil and cytarabine. There have been no previous reports of dasatinib-induced HFS in the literature. However, drugs from the same family (imatinib and lapatinib) have been documented to cause the same.[5]
Few chemotherapeutic agents are known to induce photocontact dermatitis by altering skin protection against ultraviolet exposure. Tyrosine kinase inhibitors (imatinib), c-Kit inhibitors (sunitinib and imatinib) and epidermal growth factor receptors inhibitors (such as erlotinib and vandetanib) have been documented to cause photosensitive dermatitis.[6]
The novelty of this report is that the patient developed two completely unrelated cutaneous adverse effects due to different molecules of the same family. This case highlights that timely diagnosis and appropriate management in the form of withdrawal of the offending drug is paramount while dealing with patients with an underlying malignancy to avoid delay in management.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Maitreyee Panda is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cutaneous complications of molecular targeted therapy used in oncology. J Med Life. 2016;9:19-25.
- [Google Scholar]
- Chemotherapeutic agents and the skin: An update. J Am Acad Dermatol. 2008;58:545-70.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of Bosutinib (ski-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567-76.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse reactions after treatment with dasatinib in chronic myeloid leukemia: Characteristics, potential mechanisms, and clinical management strategies. Front Oncol. 2023;13:1113462.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther. 2011;24:386-95.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatological adverse drug reactions to tyrosine kinase inhibitors: A narrative review. Clin Exp Dermatol. 2023;48:599-608.
- [CrossRef] [PubMed] [Google Scholar]





