Translate this page into:
Cutaneous photobiology
*Corresponding author: Sathish B. Pai, Professor, Department of Dermatology, Venereology and Leprology, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India. sb.pai@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Kiran, Gupta P, Pai SB. Cutaneous photobiology. Indian J Skin Allergy. 2024;3:21-7. doi: 10.25259/IJSA_11_2024
Abstract
As the outermost and largest organ of the human body, the skin is constantly exposed to solar radiation, thereby making it important to study the science of the interaction of radiation with skin called cutaneous photobiology. The electromagnetic spectrum consists of radiations of different wavelengths, but the impact of ultraviolet rays on the skin is the most important and most studied out of all. In this section, we have tried to provide a concise overview of the existing understanding of the electromagnetic spectrum, the principles of interaction of photons with skin, and its acute and chronic effects.
Keywords
Ultraviolet rays
Electromagnetic spectrum
Photoaging
Photocarcinogenesis
INTRODUCTION
The ancient Hebrews expressed the creation of the universe with the words, “And God said, “Let there be light,” and there was light.” It took countless millennia before this magnificent natural phenomenon was scientifically understood as a laboratory spectrum, a concept introduced by Sir Isaac Newton through his prism experiments.
The discovery of the ultraviolet (UV) region in the solar spectrum occurred in 1801 when Johann Ritter demonstrated a chemical reaction that was triggered by an energy present in the dark segment before the color violet. This followed Sir William Herschel’s findings in 1800, where he disclosed the presence of radiation beyond, the visible spectrum’s red end, now recognized as infrared radiation (IRR).[1]
Further understanding of the broader electromagnetic spectrum beyond the visible light (VL) was made by other scientists, including James Clerk Maxwell, Max Plank, and Heinrich Hertz, who formulated the theory of electromagnetism in the 19th century and early 20th century.[2]
ELECTROMAGNETIC SPECTRUM
The solar radiation consists of ionizing and non-ionizing radiation. Ionizing forms of electromagnetic radiation, such as X-rays or gamma rays, possess enough photon energy to completely displace an electron from an atom or molecule, causing ionization. On the contrary, non-ionizing radiation or optical radiation, encompassing UV radiation (UVR), VL, and IRR, can elevate an electron to a higher energy state.
However, unlike ionizing radiation, non-ionizing radiation cannot strip an electron from atoms or molecules.
The electromagnetic spectrum is shown in Figure 1. The electromagnetic spectrum has multiple subdivisions based on wavelength. As the wavelength increases, the energy decreases.
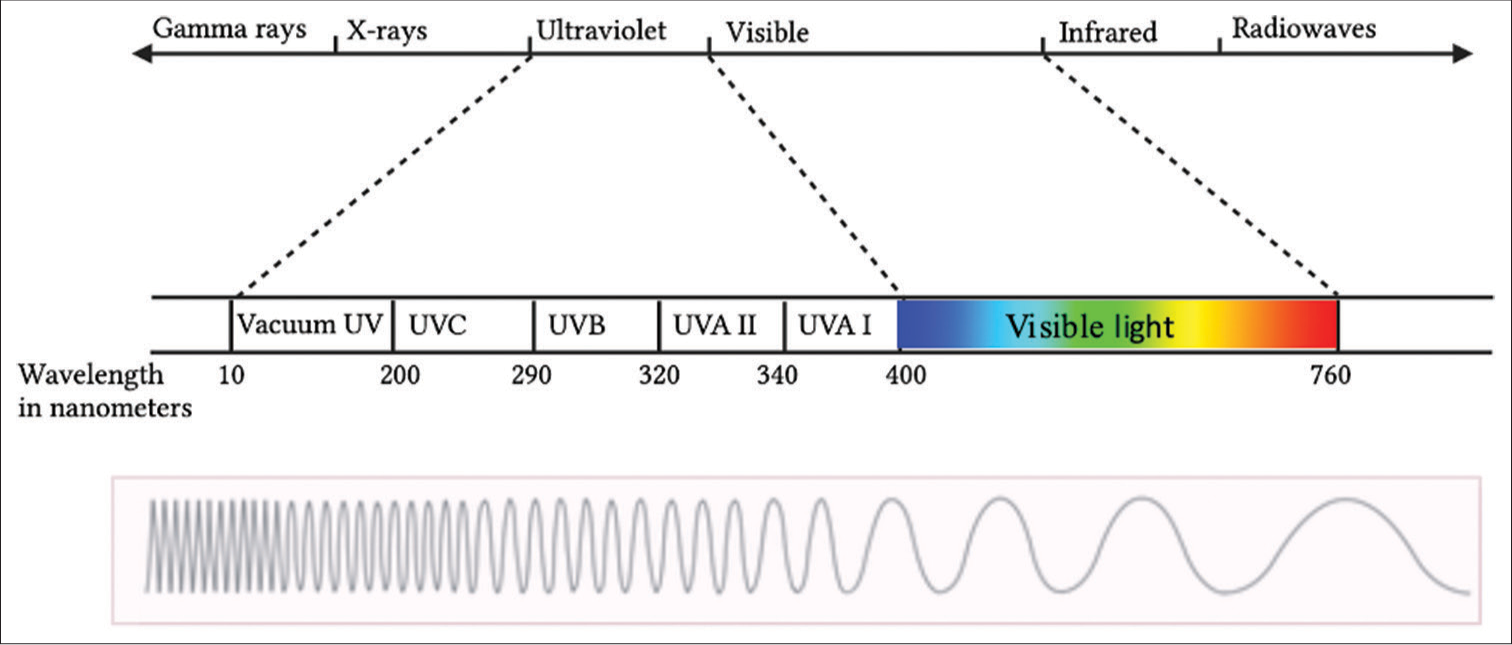
- Electromagnetic spectrum. UV: Ultraviolet, UVA: Ultraviolet A, UVB: Ultraviolet B, UVC: Ultraviolet C.
Gamma rays have the shortest wavelength and the most energy. This wavelength is usually released during nuclear reactions and is one of the ionizing radiations. X-rays are next in order between UVR and gamma rays. They reflect off a mirror and can be highly damaging.
Optical radiation, or simply, light consists of UVR, VL, and IRR. The term VL is used for the limited range of light with wavelength (400–700 nm) that is perceivable by the human eye.
UVR
The most used subdivision of UVR is the one defined by the Commission Internationale de l’Eclairage (CIE), which sets the border between ultraviolet B (UVB) and ultraviolet A (UVA) at 315 nm, compared to 320 nm in other classifications.[3] These subdivisions, however, are rather arbitrary as the photophysical properties of photons change gradually over a spectrum, rather than abruptly.
In environmental and dermatological photobiology, the commonly defined wavelength regions are as follows: UVA ranges from 400 to 320 nm, UVB from 320 to 290 nm, and ultraviolet C (UVC) from 290 to 200 nm.
The selection of 290 nm as the dividing point between UVB and UVC is made because UV radiation at shorter wavelengths is typically not found at sea level, except at elevated altitudes. Using 320 nm as the separation point between UVA and UVB is, to some extent, arbitrary. UVA has been divided further into UVA I (340–400 nm) and UVA II (320–340 nm).
LIGHT REACHING THE EARTH’S SURFACE
Around 44% of solar energy, making its way to the surface of the earth, consists of visible radiation, 45% and 5% of IRR and UVR, respectively,[4] which makes it important to understand their effects on the skin. Based on the wavelength, UVR is separated into UVA, UVB, and UVC. The stratospheric layer of the earth containing ozone filters out most of the UVC radiation. Shorter wavelengths of UVR are highly damaging, making ozone critical for life on Earth. More than 90% of UVB is also absorbed, whereas UVA travels mostly uninterrupted, and thereby it contributes about 95% of UV rays that reach the Earth’s surface.
SOURCES OF OPTICAL RADIATION
There are various natural and artificial sources of UVR, VL, and IRR. Artificial sources of light include various devices, including high-pressure xenon arc lamps, fluorescent bulbs, halogen lamps, light-emitting diodes, and lasers.[5]
Most light sources are designed to emit specific wavelengths, i.e., UVA or UVB. Nevertheless, there is unwanted emission of various other wavelengths, which can be eliminated through filters.
OPTICAL PROPERTIES OF THE SKIN
Upon entering the skin, light faces attenuation by cellular and fibrous constituents, experiencing scattering, and absorption mechanisms. The reflection and scattering of photons on and within the skin are influenced by the skin’s optical characteristics and the wavelengths of the radiation. Longer wavelengths penetrate matter deeper than shorter wavelengths.
Light interacting with the skin can either reflect or penetrate the epidermis and dermis [Figure 2]. The level of reflection of light is determined by the likelihood of reflection, which is influenced by refractive index disparities of the skin and incident angle concerning the surface vector.
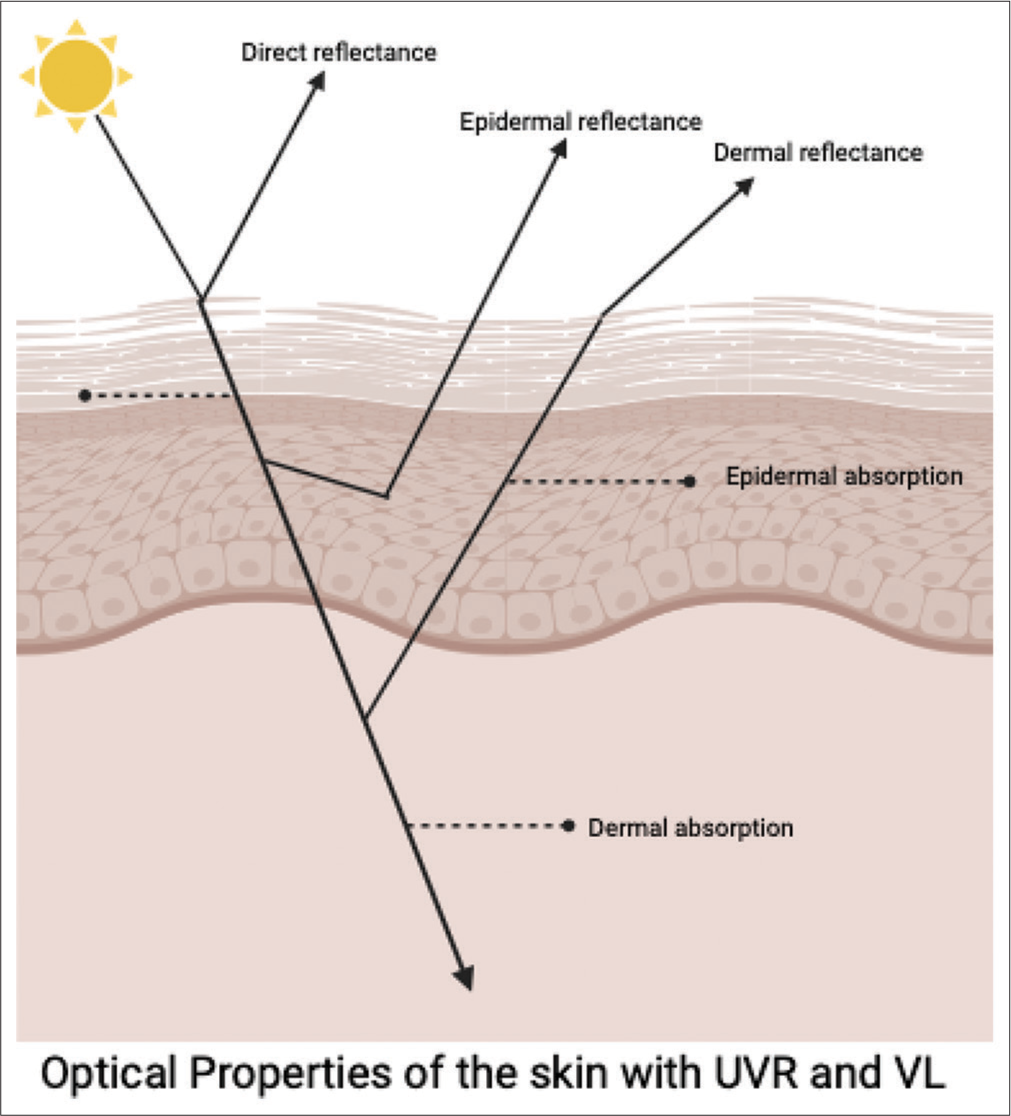
- Optical properties of the skin. UVR: Ultraviolet radiation, VL: Visible light.
Increased angles of incidence and higher refractive index on the skin surface lead to a higher reflection probability.
SCATTERING
Light can be reflected or scattered following two different principles, namely, surface scattering, which is reflection or refraction,and particulate scattering is associated with the presence of endogenous light-scattering agents such as keratin, melanin, collagen fibers, and various other smaller structures.[6,7]
This reflection occurs at three different levels depending on the
Direct reflectance (surface reflectance)
Epidermal reflectance
Dermal reflectance.
In VL, approximately 4% to –7% is estimated to be reflected into the environment from the skin surface, irrespective of the skin color or wavelength of light, while the remaining light undergoes refraction upon entering the skin.[8,9]
The filamentous proteins are the primary contributors to scattering within the skin, with keratins being the major constituents in the epidermis and collagen in the dermis, constituting around 18% to –30% of its volume.[10] Additional sources of scatter include melanosomes, cell nuclei, and walls along various skin substances present in smaller quantities. Keratinous proteins scatter light based on their orientation of fibrils within the keratin and the diameter of the fiber. The dermis contains proteins with larger diameter sizes (approximately 10 times larger than the epidermis)[10] and therefore has a higher scattering cross-section.
ABSORPTION
Light absorption in the skin is primarily influenced by hemoglobin and melanin in the visible spectrum. Hemoglobin, predominant in the dermis, acts as a major light absorber. The protein Hb A, consisting of a heme bound to 4 polypeptide chains,[11] exhibits specific peaks, with the Soret peak dominating within the blue spectrum and α and β bands contributing to the green–yellow region, collectively creating a red appearance.
Melanosomes containing melanin, situated in the epidermis, display a gradually decreasing absorption spectrum from UV to infrared. They scatter light by causing slight angular deviation in the propagation of light, as the size of the melanosomes. It has been observed that as the size of melanosomes decreases, the scattering behavior changes from that of forward scattering to a more symmetrical scattering. Melanin is primarily situated within the melanosomes in the stratum basale and is distributed upward in the epidermis on exposure to UVR, particularly UVA.
Other chromophores that contribute to the absorption of light include bilirubin, carotene, cell nuclei, and other smaller structures. These chromophores are more important for the VL spectrum whereas melanin and nucleic acids and more important for UVR.[1]
PRINCIPLES OF THE INTERACTION OF THE SKIN AND ELECTROMAGNETIC RADIATION
A series of events take place when an incident light falls on the skin, resulting in acute and chronic effects. Both acute and chronic effects are wavelength dependent.[12]
Theodor von Grotthuß and John W. Draper proposed the first law of photochemistry, according to which only the light absorbed by the irradiated matter can produce photochemical change.[13] This forms the basis of the photochemical reaction that takes place in the skin.
Chromophores are compounds that absorb light energy, resulting in various photochemical effects. Chromophores have a unique range of absorption spectrum, usually with one wavelength that is most likely to excite it, called the absorption maximum. The endogenous chromophores of the skin are deoxyribonucleic acid (DNA), melanin, urocanic acid, aromatic amino acids, flavins, and porphyrins, and exogenous chromophores include photosensitizing drugs, 8-methoxypsoralen, and sunscreens.[14-16] Table 1 mentions the maximum absorption capacity of various chromophores.[16,17]
| S. No. | Chromophore | Absorption maximum |
|---|---|---|
| 1. | DNA | 260 nm |
| 2. | Melanin | 335 nm |
| 3. | Porphyrins | 400–410 nm |
| 4. | Urocanic acid | 268 nm |
| 5. | Psoralens | 315–400 nm |
DNA: Deoxyribonucleic acid
When a photon is absorbed by a chromophore in the ground state, the electrons in the lower orbit are shifted to the higher orbit, resulting in an excited state called a singlet state.if there is no change in the spin of the electron and it is said to be in a triplet state if associated with spin change [Figure 3a and b]. The singlet state is short-lived and is in nanoseconds, whereas the triplet state is long-lived with its duration in seconds.[16,18,19]
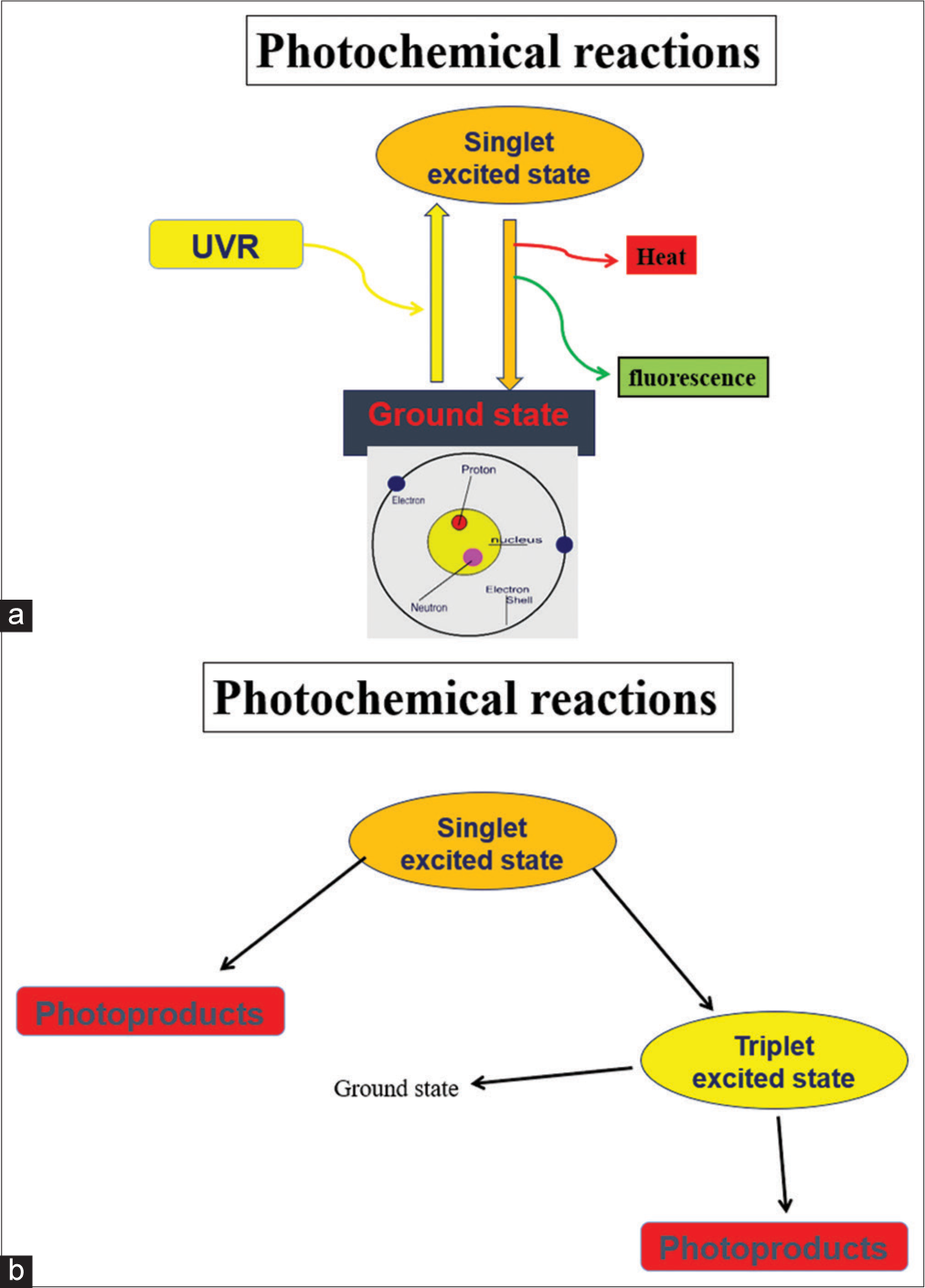
- (a and b) Mechanism of photoexcitation. UVR: Ultraviolet radiation.
Various photochemical reactions are known to occur after photoexcitation resulting in the formation of a new product called photoproduct [Figure 4]. For example, 7-dehydrocholesterol will form pre-Vitamin D3 and DNA will form cyclobutane pyrimidine dimer (CPD) upon photoexcitation.
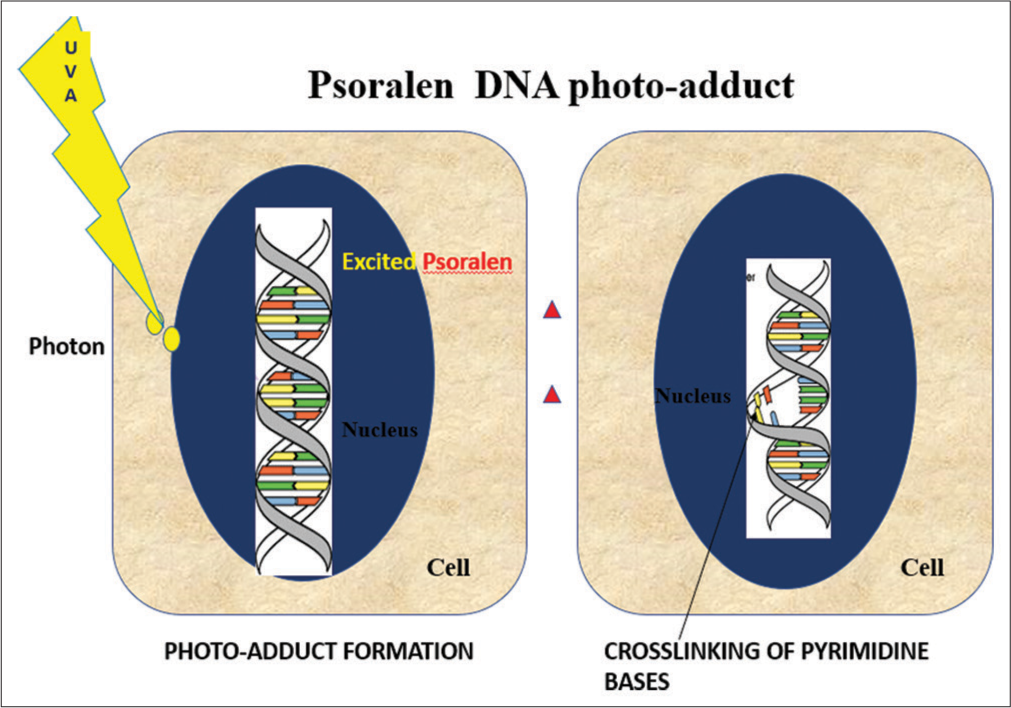
- Process of formation of photo adducts with psoralen as a chromophore. DNA: Deoxyribonucleic acid, UVA: Ultraviolet A.
A photosensitized reaction is known to occur when there is a transfer of energy from an excited chromophore to another molecule. The other scenario that can occur upon photoexcitation is that the chromophore will return to its original state by emitting energy as heat or as fluorescence rather than forming photoproducts.[16,18,19] The process is termed phosphorescence when an electron returns to the ground state from a triplet state emitting photon. In this scenario, it is worth noting that the wavelength of the emitted light is more than that of the absorbed wavelength.[16,19]
EFFECT OF UVR EXPOSURE ON THE SKIN
UVR exposure to the skin can result in acute and chronic inflammatory effects. Acute effects are sunburn, melanogenesis, and Vitamin D production, and chronic effects include photoaging and photocarcinogenesis. Considering the electromagnetic spectrum, the greater the wavelength, the deeper it will penetrate the skin but the lesser the energy. UVB, therefore, acts mainly on the epidermis; however, 10%– 15% reach the endothelial cells and microvasculature of the dermis.[20] [Figure 5] highlights the penetration depth of various wavelengths into the skin and the various cellular and cutaneous effects of UVR are tabulated in Tables 2 and 3, respectively.

- Penetration of various wavelengths of ultraviolet and visible light into the skin. UV: ultraviolet.
| 1. | Damage to DNA |
|---|---|
| 2. | Production of ROS |
| 3. | Expression of various genes and proteins |
| 4. | Melanin synthesis |
| 5. | Cell apoptosis |
| 6. | Suppression of Langerhans cell |
| 7. | Synthesis of vitamin-D |
| 8. | Nitric oxide release |
ROS: Reactive oxygen species, UV: Ultraviolet, DNA: deoxyribonucleic acid.
| Acute cutaneous changes | Chronic cutaneous changes |
|---|---|
| Erythema (sunburns) | Cutaneous malignancy |
| Photo tanning | Skin aging |
| Suppression of acquired immunity | |
| Improvement of innate immunity | |
| Lowering of blood pressure via nitric oxide | |
| Vitamin D synthesis |
UV: Ultraviolet.
EFFECT OF UVB
The acute effects of UVB are seen on the human skin as erythema along with inflammation, which is mainly neutrophilic and peaks at 24 h.[20] It is considered to be 1000 times more erythemogenic than UVA[3] owing to its shorter wavelength and therefore higher energy.
UVB is also responsible for delayed pigment tanning which is photoprotective. This occurs due to UVB associated DNA damage of keratinocytes, which upregulates p53 leading to increased levels of the pro-opiomelanocortin (POMC) gene and increased expression of α-MSH, the opioid peptide β-endorphin and adreno corticotropin hormone (ACTH) which increases melanogenesis and results in perinuclear melanin caps to protect the underlying nucleus. UVB is also associated with photoaging and has a major role in carcinogenesis.
EFFECTS OF UVA
UVA, in contrast to UVB, causes immediate pigment tanning on exposure, which is non- photoprotective. This is divided into two phases depending on the dose of UVA exposure and is based on increased melanin distribution and oxidation of melanin.[21] UVA also plays a major role in photoaging through direct epidermal damage and through the photosensitization mechanism, including the production of reactive oxygen species (ROS) (type 1) and the production of singlet oxygen (type 2).[22,23]
Table 4 enumerates the cutaneous impacts of UV rays on the skin.
| UVA | UVB |
|---|---|
| Immediate pigment darkening | Delayed melanogenesis |
| Photosensitivity reactions due to drugs | Photocarcinogenesis |
| Main contributor to photoaging Immediate erythema | Less important in photoaging 1000 times more erythemogenic; associated with sunburn |
| No vitamin D production | Role in vitamin D production |
| Penetrates glass window | Does not penetrate glass window |
| Penetrates till deep dermis | Penetrates till upper dermis |
UVA: Ultraviolet A, UVB: Ultraviolet B.
There are various photodermatoses that occur due to abnormal cutaneous response to UVR. These can broadly classify into four categories: immune-mediated dermatoses, secondary to exogenous or endogenous chemicals, defective DNA repair, and photoaggravated dermatoses. Most of the dermatoses such as polymorphic light eruption and chronic actinic dermatitis are caused by a wide range of UVR wavelengths. Solar urticaria occurs over an even broader range including UVR and VL.[24] Nutritional dermatoses like pellagra are known to be due to UVB hypersensitivity,[25] whereas photoallergic reactions are mainly due to the UVA range. Contrastingly, narrowband UVB and psoralen-based UVA therapy is also the treatment in many of these photodermatoses.
The individuals who develop these photodermatoses depend on genetic factors, susceptibility to allergens, atopic status, and photosensitivity of the skin. A commonly used scale, which is based on the amount of pigment in the skin and its reaction to solar radiation, is the Fitzpatrick scale. This scale was invented by Thomas Fitzpatrick to correlate skin color and susceptibility to melanoma. It has 6 scales ranging from I to VI where I is light skin color and burns easily and never tans and VI is dark skin which tans and does not burn easily in response to UVR.[26]
PHOTOAGING
The skin undergoes various changes as age progresses just like any other organ in the body. It is mainly due to endogenous and exogenous reasons. The endogenous skin aging is mainly due to advancing age and exogenous skin aging is due to factors such as sunlight, smoking, and other environmental pollutants. Intrinsic aging is usually a slow process, whereas external factors may fasten the process of cutaneous aging.[27-29]
PATHOMECHANISM OF PHOTOAGING
Increased oxidative stress due to an imbalance between the oxidants and antioxidants is considered the single most common reason for the pathogenesis of aging. Various oxidants are produced during the process of oxidation, metabolism, and inflammation in the body. In addition, there are external factors such as sunlight, radiation, and cigarette smoke which result in the release of oxidants.[29-31] These oxidants are taken care of by the antioxidants. The naturally occurring antioxidants are Vitamin C, Vitamin E, glutathione, lipoic acids, carotenoids, and enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione S-transferase.[32] Whenever the balance is not maintained between the two, the accumulation of oxidants will lead to aging.
The role of ROS is well established in the pathogenesis of cutaneous aging. This holds true in cases of intrinsic aging and photoaging. ROS is derived from a mitochondrial source or a non-mitochondrial source, referring to reactive molecules containing an oxygen atom, which includes free radicals and also non-radicals such as O2, peroxynitrite nitrogen reactive species (ONOO−), H2O2, and O3.[33] UVB-induced ROS activates the MAPK pathway and releases pro-inflammatory cytokines such as Interleukin (IL)-1, tumor necrosis factor (TNF)-alpha α, IL-6, intercellular adhesion molecule (ICAM-1), and cyclooxygenase (COX-2).[34]
The following changes are known to take place after UV exposure, resulting in cutaneous photoaging. There is extracellular matrix (ECM) disruption of the dermis, which is a major and the ultimate finding characterized by diminution of collagen I, III, VII, elastic fibers, and hyaluronic acid.[35,36] The functional network of fibrils, elastic fibers, glycoproteins, and glycosaminoglycans that are seen in normal skin is lost. The neutrophils may produce elastase following inflammation and UV exposure and activation of matrix metalloproteases (MMP) (MMP 1, 2, 3, and 9) in fibroblast and keratinocytes may result in ECM destruction.[33,37] In addition, the inhibitors of MMP such as TIMP1 and 3 and transforming Growth FactorTGF-β which are involved in collagen production are downregulated,and which may speed up the process of aging.[33,38] Research has established the fact that UV-induced mitochondrial DNA mutation resulted in decreased oxygen consumption leading to less ATP production in the dermal fibroblasts; and low-energy production which in turn causes mitochondrial oxidative stress and photoaging.[39,40]
PHOTOCARCINOGENESIS
UVR can affect various cellular components such as DNA, Ribonucleic acid (RNA), and proteins by direct and indirect processes. There are three major mechanisms by which UV rays are known to play a role in cutaneous photocarcinogenesis.[41]
After absorbing UVB rays, CPDs and pyrimidine–pyrimidone (6–4) photoproducts are formed with the help of a bond between pyrimidine bases and their adjacent counterpart of DNA [Figure 6].[42] Further, UVR on the unstable 6–4 pyrimidine–pyrimidone or 6–4 pyrimidine–pyrimidinone will convert these molecules into still more unstable forms called Dewar valence isomers.[43] The UV rays-induced CPDs (thymine-cytosine [T=C] and cytosine-cytosine [CC] dimmers) are considered to be oncogenic because of their similarity to tumor suppressor P53 gene present in the tumor suppressor P53 gene.[44] All the above-said three mechanisms will result in UV-specific changes. The body has its mechanism for repairing these changes called nucleotide excision repair (NER). If this mechanism is faulty, these changes will result in mutation predisposing to various cancers.[45,46]

- Depiction of the effects of ultraviolet rays on deoxyribonucleic acid and Langerhans cell, UV: Ultraviolet, UVB: Ultraviolet B, DNA: Deoxyribonucleic acid, UCA: Urocanic acid, ROS: Reactive oxygen species, CPD: Cyclobutane pyrimidine dimer, P:Phosphate.
Not just DNA, messenger RNA, and other types of RNA can also result in various biological changes upon UV exposure, like the formation of dysfunctional proteins. Research has proved that triplet states of tryptophan and tyrosine ultimately result in superoxide anions (O2-.) formation. Modified proteins may form aggregates within the cell and may prove to be damaging; resulting in skin aging and carcinogenesis.[47] UV rays also accelerate insulin-like growth factor 1 (IGF-1) expression, which in turn stimulates keratinocyte epidermal IGF-1 receptor; thereby, keratinocytes become resistant to apoptosis. This reason in combination with the reactive oxidative stress is known to increase the risk of various skin cancers such as basal cell epitheliomas and squamous cell carcinomas in aged skin.[48]
CONCLUSION
Knowing cutaneous photobiology in depth will help us understand the pathomechanism of photodermatitis and cutaneous carcinomas. Further studies to know the effect of VL and infrared light on the skin are warranted, thereby validating the use of sunscreens filtering those wavelengths. Exploring the diverse spectrum of the skin types in India presents a captivating avenue for analysis, considering the rich tapestry of ethnicities and climatic variations across the subcontinent.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Dr. Sathish Pai is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- What is light? Photodermatol Photoimmunol Photomed. 2002;18:68-74.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous photobiology: Past, present and future. J Invest Dermatol. 1976;67:209-24.
- [CrossRef] [PubMed] [Google Scholar]
- Structure and function of skin In: Fitzpatrick's dermatology (9th ed). New York: McGraw-Hill Education; 2019. p. :265-9.
- [Google Scholar]
- Solar ultraviolet radiation at the earth's surface. Photochem Photobiol. 1989;50:443-50.
- [CrossRef] [Google Scholar]
- Light-based therapies for skin of color In: Light-based therapies for skin of color. London: Springer; 2009. p. :1-284.
- [CrossRef] [Google Scholar]
- Optical properties of skin surface In: Agache's measuring the skin: Non-invasive investigations, physiology, normal constants (2nd ed). United States: Springer International Publishing; 2017. p. :85-98.
- [Google Scholar]
- Optical properties of human skin. J Biomed Opt. 2012;17:909011.
- [CrossRef] [PubMed] [Google Scholar]
- Optical radiation transfer in the human skin and applications in in vivo remittance spectroscopy In: Proceedings of the symposium on bioengineering and the skin, Cardiff, Wales, 1979. London: MTP Press Ltd; 1980.
- [CrossRef] [Google Scholar]
- UV-A: Biologic effects of ultraviolet radiation with emphasis on human responses to Longwave ultraviolet. New York: Plenum Press; 1978.
- [CrossRef] [Google Scholar]
- Chapter 3: Anatomy and organization of human Skin In: Rook's textbook of dermatology Vol 1. (7th ed). Massachusetts, CA: Blackwell Publishing; 2004. p. :3.3-3.80.
- [Google Scholar]
- Chapter 7: Hemoglobin: Portrait of a protein in action In: Biochemistry (6th ed). New York: WH Freeman; 2006. p. :183-94.
- [Google Scholar]
- Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978-86.
- [CrossRef] [PubMed] [Google Scholar]
- Chromophore activation of α,β-unsaturated carbonyl compounds and its application to enantioselective photochemical reactions. Angew Chem Int Ed Engl. 2018;57:14338-49.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-9.
- [CrossRef] [PubMed] [Google Scholar]
- Tracing the photoaddition of pharmaceutical psoralens to DNA. Molecules. 2020;25:5242.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraweak photon emission induced by visible light and ultraviolet a radiation via photoactivated skin chromophores: In vivo charge coupled device imaging. J Biomed Opt. 2012;17:85004.
- [CrossRef] [PubMed] [Google Scholar]
- TNF-a and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: Correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763-8.
- [CrossRef] [PubMed] [Google Scholar]
- Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp Dermatol. 2021;30:560-71.
- [CrossRef] [PubMed] [Google Scholar]
- Actions of ultraviolet light on cellular structures. EXS. 2006;96:131-57.
- [CrossRef] [PubMed] [Google Scholar]
- Solar urticaria: The annoying photodermatosis. Int J Dermatol. 1999;38:411-8.
- [CrossRef] [PubMed] [Google Scholar]
- Skin typing: Fitzpatrick grading and others. Clin Dermatol. 2019;37:430-6.
- [CrossRef] [PubMed] [Google Scholar]
- Research progress on skin photoaging and oxidative stress. Postepy Dermatol Alergol. 2021;38:931-6.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:33-6.
- [Google Scholar]
- Role of antioxidants in the skin: Anti-aging effects. J Dermatol Sci. 2010;58:85-90.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in the skin: Impact and related protection. Int J Cosmet Sci. 2021;43:495-509.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidants and the skin: Understanding formulation and efficacy. Dermatol Ther. 2012;25:252-9.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in aging human skin. Biomolecules. 2015;5:545-89.
- [CrossRef] [PubMed] [Google Scholar]
- Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev Med Chem. 2011;11:1200-15.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced type I and type III procollagens in photodamaged adult human skin. J Investig Dermatol. 1995;105:285-90.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features of photodamaged human skin are associated with a reduction in collagen VII. Br J Dermatol. 1997;137:344-50.
- [CrossRef] [PubMed] [Google Scholar]
- Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197-250.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123:801-10.
- [CrossRef] [PubMed] [Google Scholar]
- Role of mitochondria in photoaging of human skin: The defective powerhouse model. J Investig Dermatol Symp Proc. 2009;14:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T) Front Bioeng Biotechnol. 2020;8:919.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: A comprehensive review. Heliyon. 2023;9:e13580.
- [CrossRef] [PubMed] [Google Scholar]
- Formation of cyclobutane pyrimidine dimers at dipyrimidines containing 5-hydroxymethylcytosine. Photochem Photobiol Sci. 2013;12:1409-15.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of cyclobutane pyrimidine dimers, pyrimidine (6-4) pyrimidone photoproducts, and Dewar valence isomers by natural sunlight in normal human mononuclear cells. Cancer Res. 1995;55:2245-8.
- [Google Scholar]
- Redox control of the ubiquitin-proteasome system: From molecular mechanisms to functional significance. Antioxidants Redox Signal. 2011;15:2265-99.
- [CrossRef] [PubMed] [Google Scholar]
- Insight in DNA repair of UV-induced pyrimidine dimers by chromatographic methods. Photochem Photobiol. 2017;93:207-15.
- [CrossRef] [PubMed] [Google Scholar]
- Photoimmunology and nucleotide excision repair: Impact of transcription coupled and global genome excision repair. J Photochem Photobiol B. 2001;65:97-100.
- [CrossRef] [PubMed] [Google Scholar]
- Actions of ultraviolet light on cellular structures In: Cancer: Cell structures, carcinogens and genomic instability. Germany: Springer; 2006. p. :131-57.
- [CrossRef] [PubMed] [Google Scholar]
- The IGF-1/IGF-1R signaling axis in the skin: A new role for the dermis in aging-associated skin cancer. Oncogene. 2010;29:1475-85.
- [CrossRef] [PubMed] [Google Scholar]







