Translate this page into:
Comorbidities in patients with chronic urticaria: A cross sectional study from an Urticaria Center of Reference and Excellence
*Corresponding author: Rossy Moreira Bastos Junior, Department of Immunology, Hospital Universitário Clementino Fraga Filho (HUCFF-UFRJ), Rio de Janeiro, Brazil. dr.rossymbastos@uol.com.br
-
Received: ,
Accepted: ,
How to cite this article: Bastos RM Jr., Dortas SD Jr., Fernandes AC, Azizi GG, Lupi O, Valle SO. Comorbidities in patients with chronic urticaria: A cross sectional study from an Urticaria Center of Reference and Excellence. Indian J Skin Allergy. 2024;3:106-10. doi: 10.25259/IJSA_26_2023
Abstract
Objectives:
Chronic urticaria (CU) is a common mast cell driven disease, characterized by the development of wheals, angioedema, or both. The common comorbidities among patients of CU presenting to an Urticaria Center of Reference and Excellence (GA2LEN UCARE) in Rio de Janeiro, Brazil are presented.
Material and Methods:
We conducted a cross-sectional and single-center study with adolescents and adults from the outpatient clinic of a GA2LEN UCARE Center at the Immunology Service. Patients were enrolled after informed consent was obtained.
Results:
We enrolled 180 patients with CU. One hundred and fifty-five were female (86.1%) and 25 male (13.9%). Mean age was 46.2 ± 16.1 years (ranging from 13 to 81 years). Mean disease duration was 10.3 years (ranging from 0.17 to 62 years). The most frequent comorbidities associated with CU were hypertension in 63 patients (35%), atopy 58 (32.2%), thyroid disease 34 (18.8%), gastrointestinal disease 25 (13.8%), diabetes 22 (12.2%), psychiatric disorders 22 (12.2%), rheumatic diseases 17 (9.4%), and hepatitis C (2. 2%).
Conclusion:
CU has been related to several comorbidities. Our data matches previous reported findings regarding sex, age, and comorbidities such as autoimmunity, atopy, and hypertension. It is necessary to improve the diagnosis of comorbidities. With the early diagnosis of comorbidities, we will be able to carry out timely therapeutic interventions to improve the effectiveness of the treatment and ensure safety in drug interactions.
Keywords
Chronic urticaria
Comorbidities
Chronic spontaneous urticaria
Chronic inducible urticaria
Urticaria
INTRODUCTION
Chronic urticaria (CU) is a mast cell-driven disease characterized by the development of wheals, angioedema, or both for ≥6 weeks. Disease is divided into chronic spontaneous (i.e., with spontaneous onset of symptoms) urticaria (CSU) and chronic inducible (i.e., with symptoms triggered by reproducible specific triggers such as cold or pressure) chronic inducible urticaria (CIndU). It affects about 1% of the world population of all ages, mostly young and middle-aged women. The average duration of CU is about five years. However, in up to 30% of the patients, the symptoms may persist for more than five years.[1-4]
Comorbidities are common and may complicate treatment response, as well as negatively impact patients’ quality of life (QoL). CU has been related to comorbidities such as autoimmunity, psychiatric, and atopic diseases, all of which are strongly overrepresented among CU patients. It is not clear if malignancies, cardiovascular, and gastrointestinal diseases coexist with CU.[5,6]
Many studies have investigated the association of autoimmune comorbidities with CU. It is observed that not only the frequency but also the risk of presenting with autoimmune diseases is increased in patients with CU.[7] In a systematic review of the literature, the most common autoimmune comorbidities associated with CU were autoimmune thyroid diseases and vitiligo.[8,9]
In addition, a higher prevalence of type I diabetes mellitus (1.8%), vitiligo (0.4%), and celiac disease,and rheumatoid arthritis (0.6%) has also been reported.[10] Among the autoimmune diseases, thyroid disorders have been the most commonly encountered diseases among patients with CU, with a reported prevalence ranging up to more than 50%, depending on the inclusion criteria.[7,11]
Ghazanfar et al. demonstrated that in addition to autoimmune diseases, there is an increased prevalence of atopic diseases in patients with CU, including atopic dermatitis, asthma, and rhinoconjunctivitis.[12] Previously, a high prevalence of psychiatric comorbidities was demonstrated in patients with CU, ranging up to 60%. Anxiety, depression, and somatoform disorders with significant negative impact on QoL were demonstrated in more than 30% of the patients.[12-14]
Cardiovascular diseases are other disorders associated with CU in high frequency. Among them, systemic arterial hypertension was the most common disease, but these data were not relevant in the correlation with the risk of developing the same disease.[6-8]
The aim of this study was to describe common comorbidities among patients with CU at a Brazilian Urticaria Center of Reference and Excellence (GA2LEN UCARE).[15]
MATERIAL AND METHODS
A single-center and cross-sectional study was carried out among adolescents and adults from the outpatient clinic of a Reference and Excellence Center in Urticaria (GA2LEN UCARE, www.ga2len-ucare.com) in the Immunology Service of a university hospital. Patients were enrolled after obtaining informed consent. The patients were selected at the CU outpatient clinic, and followed up at the allergy and immunology service at Clementino Fraga Filho University Hospital (HUCFF-UFRJ). Data were taken from these protocols after systematically reviewing each file. This study was approved by the Research Ethics Committee HUCFF-UFRJ.
RESULTS
We enrolled 180 patients with CU. One hundred and fifty-five were female (86.1%),and 25 male (13.9%). Mean age was 46.2 ± 16.1 years (ranging from 13 to 81 years). Mean disease duration was 10.3 years (ranging from 0.17 to 62 years) [Table 1]. The association between CSU and CindU was found in 95 of the study patients (79.7%). Elevated Immunoglobulin E (Ig E; >100IU/ml) was detected in 47% patients. Autologous serum skin test (ASST) was positive in 45.6% of our patients. In addition, patients with angioedema had ASST positivity in 72.6%, that was associated with a higher UASF score. The association between CSU and CIndU occurred in more than half of our patients.
| Gender | (n of patients/%) |
| Females | 155 (86.1) |
| Males | 25 (13.9) |
| Age±standard deviation, range (in years) | 46.2±16.1, 13–81 |
n: Number of patients.
The most frequent comorbidities associated with CU were hypertension in 63 patients (35%), atopic diseases or type 1 allergy such as rhinitis, asthma and atopic dermatitis 58 (32.2%), thyroid disease in 34 (18.8%), gastrointestinal disease in 25 (13.8%), diabetes in 22 (12.2%), psychiatric disorders in 22 (12.2%), rheumatic diseases in 17 (9.4%), and hepatitis C (2.2%) [Figure 1]. Thirty-seven (20.5%) patients presented with other comorbidities, such as dyslipidemia, glaucoma, chronic obstructive pulmonary disease, epilepsy, thrombophilia, sarcoidosis, amyloidosis, and vitiligo. Six of our patients presented with malignancies (cervical, bladder, breast, colon and Hodgkin’s lymphoma).
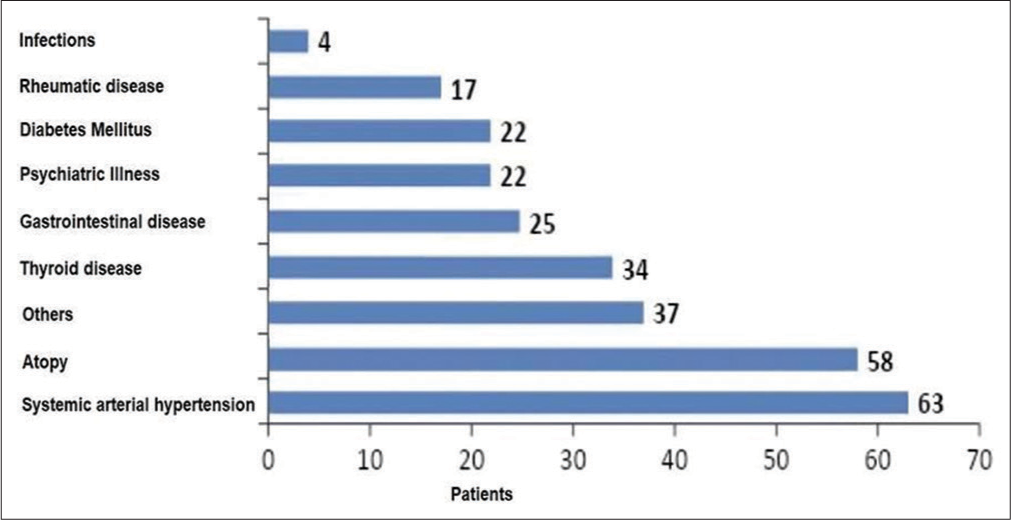
- Most frequent comorbidities in patients.
DISCUSSION
CU significantly influences the quality of life (QOL) of patients. Concomitance with other comorbidities can lead to greater morbidity. CU is a self-limited disease with a higher incidence in younger adults; in many cases, it may persist into adulthood, adding to other age-related comorbidities, thus further compromising the QoL of these patients. When compared to other diseases, CU, atopic dermatitis, and psoriasis share several comorbidities,with cardiovascular and psychiatric disorders being the most prevalent.[16,17]
The study population had a characteristically typical distribution of patients with CU, such as a higher proportion of female patients (86.1%); most with CSU and concomitant CIndU. Several epidemiologic studies indicated that the prevalence of CU is at least twice as high in female patients compared with male patients. Female patients seem to seek medical care more often than men, as they are often more affected by autoimmune diseases.[18,19] Recently, two patient-related outcome measures studies in CU performed at our outpatient clinic showed 82% female patients.[20-24]
Our data shows that the most frequent comorbidity associated with CU was hypertension documented in 63 patients (35%), significantly lower when compared to 42.9% found by Weller et al.[7] This finding could possibly be related to the indiscriminate use of corticosteroids, often as self medication by the patients. Few studies have shown that hypertension is associated with increased duration of CU, a fact that was not observed in our population. Similar to hypertension, the occurrence of diabetes in CU is probably due to the indiscriminate use of glucocorticoids.[6,8] Ghazanfar et al. found 2.3% of CU patients presenting with diabetes.[25] We believe that in our study, the amount of patients with diabetes (12,2%) was due to the frequent and lasting use of systemic corticosteroids, despite lack of any recommendations for use of corticosteroids by any international guidelines.
The association of autoimmunity is concurrent with that of other studies. Around 18.8% of the patients were diagnosed with autoimmune thyroid disease in this study. This was slightly higher when compared with other studies that had a prevalence ranging from 13% to 15%, data that reaffirms that autoimmunity is closely related to the pathogenesis of CU. At the VI International Congress of Dermatology in New York, Ravitch first suggested an association between CSU and thyroid disease. In some studies, the presence of thyroid autoantibodies was found in up to 50% of cases. In line with the literature, in our study also, this was the most common autoimmune comorbidity in patients with CU. The most common circulating autoantibodies in CU patients are anti-TPO, in addition to other antibodies.[26-29] The impact of CU symptoms and therapeutic interventions in patients with concomitant thyroid disease, may contribute to a more severe impairment in QoL of patients, especially due to the chronic nature of the disease.[30-33]
Other autoimmune comorbidities that were frequently associated include rheumatic diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus, detected in approximately 6% of our patients. Rheumatoid arthritis is a disease of high complexity that promotes inflammation and joint damage, causing physical and work disabilities, emotional and financial losses, and impacting the QoL. Further studies are required regarding the prevalence and risk of developing autoimmune diseases in our CU patients.[34-37]
Although CU, particularly CSU, is not an atopic condition, several studies show atopic comorbidities associated with CU, as shown in an Israeli study.[18] There may be a high frequency of atopic comorbidities in the general population of CU.[19]
Our data revealed that atopic diseases were also over represented amongst our CU patients being detected in 32.2%. Gonçalo et al. described an association between atopy and CU in about 16.9% of patients.[3] Among the most frequently detected atopic diseases in the literature, include atopic dermatitis, asthma, and rhinoconjunctivitis.[37] Further studies should be conducted that correlate this high frequency of atopic comorbidities in CU patients.[38]
This data is correlated with atopy, although papular and CU angioedema are rarely associated with exposure to allergens. This is despite the possible association between urticaria and presentation with severe allergies in emergency care, as described in several articles.[39,40]
IgE levels were formerly compared to the time of evolution of the disease. Normal levels of IgE may be possible with longer duration of treatment, disease progression, in addition to higher UAS7 scores and impaired QoL (Cu-Q2oL). There is also an association of low IgE levels with low therapeutic response. These data are being evaluated in a study in our service. We believe that the physical, emotional, social, and financial impact of these atopic diseases and CU on patients and families is very significant. Classically, itching and sleep disturbances are the most described symptoms that impact patients globally.[38] A higher risk of patients with CSU having mastocytosis and anaphylaxis has been reported in literature. This was not evident in our patients.[41]
Malignancy was found in 6 (9%) of our patients. This finding should lead us to consider the investigation of malignancy mainly in patients with poor response to the recommended conventional treatment. There are a few reports of the association between CU and malignancy in the literature.[42-44]
Psychiatric disorders were present in 12.2%, which was slightly lower when compared to other studies. Psychiatric disorders are a frequent comorbidity, mainly anxiety, which has a strong impact on patients’ QoL.[16] Chu et al. detected psychiatric disorders (PD) in 8.53% of patients with CU, lower than our data. In contrast, Staubach and Juhlin reported a much higher prevalence of 35% and 16 % respectively.[2,16,45] In contrast, the prevalence of psychiatric disorders in our patients was much lower when compared to the study of the group in Portugal, who documented it in 16% of their patients.[46] As with atopic dermatitis and psoriasis, having a chronic skin disease such as CU is considered an important factor when it comes to mental health and QoL for these patients. Lack of sleep due to itching is one of the most likely reasons related to sleep disorders, but also the hypothesis of the inflammatory process itself in the genesis of psychiatric symptoms. The overall prevalence of any psychiatric comorbidity among CU patients was estimated at 31.6% by Konstantinou et al. The most prevalent psychiatric disorders were sleep-wake disorders and anxiety disorders; other alterations found were anxiety disorders, mood disorders, trauma, depression, and stress-related disorders.[34,45,47,48]
We believe that higher prevalence and increased risk of depression may be due to delay in the diagnosis, which often lasts for years, due to visits to many professionals with different specialties. The visible skin lesions themselves can generate embarrassment, and difficulties in social life. The long duration of the disease, the difficulty in identifying the causes and/or triggers, and, mainly, the unsatisfactory response to currently available treatments are reasons that other studies also share as a possible genesis of these disorders. Psychological health, pruritus, and sleep loss are therefore important parameters to be evaluated during consultations in patients presenting with CU.
Association with infections was also evaluated in our study, but not directed to an etiologic agent; changes in the blood count with signs of infection were detected in approximately 2.2% of the patients, data similar to those described by other authors. Some studies establish an unclear link between CSU and Helicobacter pylori infection, parasitic infections, and chronic viral infections, including hepatitis B and C virus and human herpesvirus 6.[33,49-52]
CONCLUSION
CU has been related to several comorbidities. Our data matches previous reported findings regarding sex, age, and comorbidities such as autoimmunity, atopy, and hypertension. Coexisting disorders such as autoimmune diseases, atopy, and psychiatric disorders are common in the population studied. Considering the small sample size and single center study, further studies are warranted.
Ethical approval
This study was approved by the Research Ethics Committee of the Clementino Fraga Filho University Hospital (HUCFF-UFRJ) number (CAAE 88430318.0.0000.525), dated on 07/07/2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil
References
- Chronic urticaria in the real-life clinical practice setting in Sweden, Norway and Denmark: Baseline results from the noninterventional multicentre AWARE study. J Eur Acad Dermatol Venereol. 2017;31:1048-55.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic urticaria treatment patterns and changes in quality of life: AWARE study 2-year results. World Allergy Organ J. 2020;13:100460.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226-36.
- [CrossRef] [PubMed] [Google Scholar]
- Refractory chronic urticaria in adults: Clinical characterization and predictors of severity. Allergy Asthma Clin Immunol. 2020;16:97.
- [CrossRef] [PubMed] [Google Scholar]
- World Allergy Organ J. 2021;14:100533.
- [CrossRef] [PubMed]
- Cardiovascular risk is not increased in patients with chronic urticaria: A retrospective population-based cohort study. Acta Derm Venereol. 2017;97:261-2.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, comorbidities, and healthcare utilization of patients with chronic urticaria in Germany. J Eur Acad Dermatol Venereol. 2022;36:91-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of arterial hypertension on chronic urticaria duration. Ann Allergy Asthma Immunol. 2009;103:407-10.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: A systematic review. Allergy. 2017;72:1440-60.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun Rev. 2017;16:1196-208.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of chronic spontaneous urticaria in patients with autoimmune thyroid diseases: A nationwide, population-based study. Allergy Asthma Immunol Res. 2017;9:373-7.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of comorbidities in patients diagnosed with chronic urticaria: A nationwide registry-study. World Allergy Organ J. 2020;13:100097.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy. 2017;72:2005-16.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91:557-61.
- [CrossRef] [PubMed] [Google Scholar]
- Definition, aims, and implementation of GA(2)LEN urticaria centers of reference and excellence. Allergy. 2016;71:1210-8.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in patients with chronic urticaria is differentially impaired and determined by psychiatric comorbidity. Br J Dermatol. 2006;154:294-8.
- [CrossRef] [PubMed] [Google Scholar]
- Psychiatric disorders associated with some chronic dermatologic diseases among a group of Egyptian dermatology outpatient clinic attendants. J Egypt Womens Dermatol Soc. 2017;14:31-6.
- [CrossRef] [Google Scholar]
- Characterization of chronic urticaria and associated conditions in a large population of adolescents. J Am Acad Dermatol. 2019;81:129-35.
- [CrossRef] [PubMed] [Google Scholar]
- IgE mediated autoallergy against thyroid peroxidase-a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794.
- [CrossRef] [PubMed] [Google Scholar]
- Validity, reliability, and interpretability of the Brazilian urticaria control test. Allergy Asthma Proc. 2020;41:e61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Spiritual well-being and quality of life are impaired in chronic urticaria. Eur Ann Allergy Clin Immunol. 2021;53:221-7.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic idiopathic urticaria: Comparison of the clinical features of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Am Acad Dermatol. 1999;40:443-50.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic idiopathic and chronic autoimmune urticaria: Clinical and immunopathological features of 68 subjects. Acta Derm Venereol. 2004;84:288-90.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy. 2020;75:423-32.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of comorbidities in patients diagnosed with chronic urticaria: A nationwide registry-study. World Allergy Organ J. 2020;13:100097.
- [CrossRef] [PubMed] [Google Scholar]
- The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-34.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologic aspects of Hashimoto's thyroiditis and Graves' disease in Rochester, Minnesota (1935-1967), with special reference to temporal trends. Metabolism. 1972;21:197-204.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252-65.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus-a systematic review. Clin Exp Allergy. 2016;46:275-87.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid hormone and its metabolites in relation to quality of life in patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf). 2016;85:781-8.
- [CrossRef] [PubMed] [Google Scholar]
- Provocation tests with antiphlogistica and food additives in recurrent urticaria. Dermatologica. 1975;151:360-7.
- [CrossRef] [PubMed] [Google Scholar]
- A descriptive study of clinical-epidemiological profile of chronic urticaria from tertiary care center. Int J Res Dermatol. 2021;7:184-7.
- [CrossRef] [Google Scholar]
- EAACI taskforce position paper: Evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune thyroid disease and rheumatoid arthritis: Relationship and the role of genetics. Immunol Res. 2014;60:193-200.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646-56.
- [Google Scholar]
- How does one assess early rheumatoid arthritis in daily clinical practice? Best Pract Res Clin Rheumatol. 2001;15:67-76.
- [CrossRef] [PubMed] [Google Scholar]
- Out of the skin of babes: Measuring the full impact of atopic dermatitis in infants and young children. J Invest Dermatol. 2012;132:2494-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mediators of inflammation and angiogenesis in chronic spontaneous urticaria: Are they potential biomarkers of the disease? Mediators Inflamm. 2017;2017:4123694.
- [CrossRef] [PubMed] [Google Scholar]
- Activation markers CD63 and CD203c are upregulated in chronic urticaria. Ann Dermatol. 2013;25:522-3.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, prognosis, and risk factors in mastocytosis. Immunol Allergy Clin North Am. 2014;34:283-95.
- [CrossRef] [PubMed] [Google Scholar]
- Very rarely chronic urticaria can be caused by cancer and if so, resolves with its cure. Allergy. 2018;73:1925-6.
- [CrossRef] [Google Scholar]
- Urticaire chronique superficielle associée aux cancers solides: Un cas et revue de la littérature [Chronic superficial urticaria associated with solid cancers: Case report and literature review] Ann Dermatol Venereol. 2019;146:377-81.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and comorbidities of patients with chronic urticaria in Taiwan: A nationwide population-based study. J Dermatol Sci. 2017;88:192-8.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent urticaria: Clinical investigation of 330 patients. Br J Dermatol. 1981;104:369-81.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic spontaneous urticaria in children-a systematic review on interventions and comorbidities. Pediatr Allergy Immunology. 2018;29:303-10.
- [CrossRef] [PubMed] [Google Scholar]
- The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
- [CrossRef] [PubMed] [Google Scholar]
- The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS One. 2018;13:e0207602.
- [CrossRef] [PubMed] [Google Scholar]
- Serological evidence that activation of ubiquitous human herpesvirus-6 (HHV-6) plays a role in chronic idiopathic/spontaneous urticaria (CIU) Clin Exp Immunol. 2016;183:230-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association between urticaria and virus infections: A systematic review. Allergy Asthma Proc. 2016;37:18-22.
- [CrossRef] [PubMed] [Google Scholar]
- Headache deteriorates the quality of life in children with chronic spontaneous urticaria. Allergol Immunopathol (Madr). 2019;47:254-9.
- [CrossRef] [PubMed] [Google Scholar]







