Translate this page into:
Abrocitinib: A comprehensive review of its use in dermatology beyond atopic dermatitis
*Corresponding author: Arunima Ray, Department of Dermatology, Narayana Health - Rabindranath Tagore Institute of Cardiac Sciences, Kolkata, West Bengal, India. arunima.roma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ray A, Pandhi D. Abrocitinib: A comprehensive review of its use in dermatology beyond atopic dermatitis. Indian J Skin Allergy. 2025;4:25-42. doi: 10.25259/IJSA_54_2024
Abstract
Janus kinase (JAK) inhibitors are small molecule inhibitors that restrict proinflammatory pathways and are used in various autoimmune and inflammatory conditions, thus gaining significant threshold in dermatology. Abrocitinib, a JAK 1 inhibitor, was first approved in 2021 for use in atopic dermatitis (AD) and has been shown to be effective and safe in most cases. Since its approval, abrocitinib has also been reported to be varyingly successful in a group of other dermatological conditions and is a relatively safe drug. Our comprehensive review is based on an extensive PubMed search of all published literature on the use of abrocitinib in dermatological disorders with the exclusion of AD whereby we identified and included 37 papers. These include diverse eczematous, autoimmune, inflammatory, and keratinization disorders. Beyond AD, the largest number of patients were reported for vitiligo, hand eczema, chronic pruritus, and prurigo nodularis, all of which reported favorable outcomes. Pharmacodynamic studies have shown a dose-dependent decrease in platelet counts and disruption in lipid levels. Other mild adverse effects include nausea and dizziness which do not merit drug discontinuation. Our review highlights the broad usefulness that abrocitinib has as a therapeutic agent in inflammatory and autoimmune dermatoses, often when used as a single therapeutic agent, and has predictable safety and tolerability profiles. A larger number of randomized controlled trials are required to validate the off-label uses of abrocitinib and to optimize dosing strategies. This review also includes information about dosing recommendations, drug monitoring, and the use of oral abrocitinib in special patient groups.
Keywords
Abrocitnib
Eczema
Janus kinase inhibitors
Janus kinase inhibitors in dermatology
INTRODUCTION
The inflammatory cytokine response is mediated by the interaction between the Janus kinase–signal transducers and activators of transcription of the JAK-STAT pathway. The family of JAK includes 4 protein members of the JAK 1/2/3 and tyrosine kinase 2 and the family of STAT includes the STAT 1/2/3/4/5A/5B/6.
When activated, the JAK mediators pair within themselves and set in motion a downstream inflammatory cascade. The cytokine receptors phosphorylate the associated JAKs, with subsequent phosphorylation of the STAT proteins. When the STATs dimerize, they then translocate to the cellular nucleus with subsequent transcription of the genes. A dysregulated pattern of these proinflammatory JAK-STAT pathways has been seen in autoimmune and inflammatory dermatoses.
The development of JAK inhibitors ensured that these agents act on a specific target for curtailing inflammatory processes and suppressing immune-specific responses.[1]
This has led to the development of a host of small molecule inhibitors that act on various components of the JAK-STAT pathway, and there is a growing incorporation of these agents in the treatment of various dermatological conditions.
One such JAK1 inhibitor is abrocitinib (ab “roe sye” ti nib) which has been gaining popularity in the pharmacological arena, with its first approval in the UK in 2021 [Figure 1].[2] Prior to the approval of the drug in the UK, various Phase 3 studies under JAK1 Atopic Dermatitis Efficacy and Safety (JADE) trials and clinical trials were conducted with abrocitinib [Figure 2].[3]
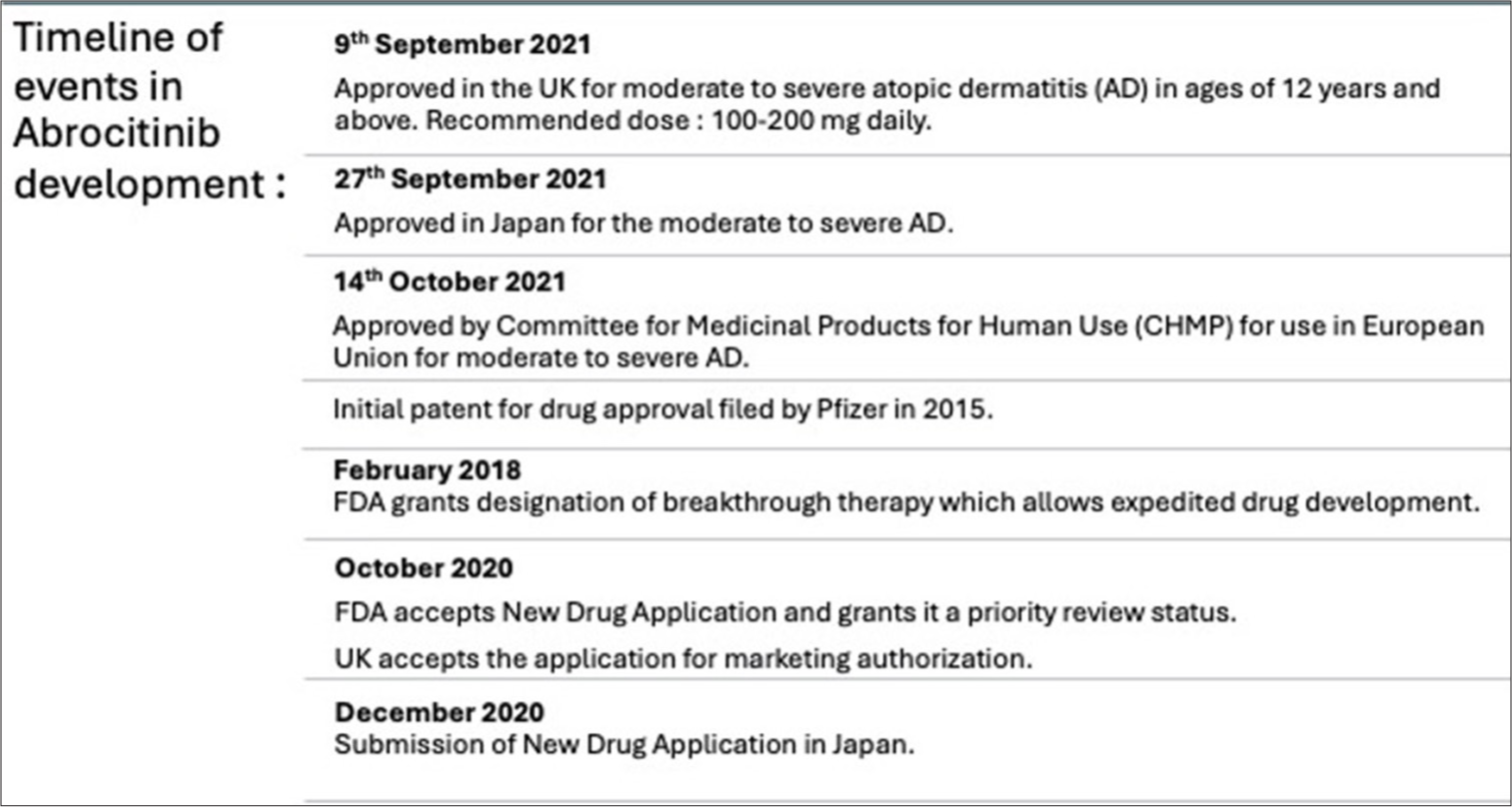
- Timeline of development of abrocitinib. FDA: Food and drug administration.

- Janus kinase 1 atopic dermatitis efficacy and safety trials to assess the efficacy of abrocitinib in atopic dermatitis. AD: Atopic dermatitis, JADE: Janus kinase 1 atopic dermatitis, CNI : Calcineurin Inhibitors.
MATERIALS AND METHODS
This review aims to evaluate the available scientific literature to understand the reported off-label uses of abrocitinib in dermatological conditions including atopic dermatitis (AD) and beyond. The authors conducted this review to summarize the literature on using abrocitinib for treating dermatological conditions including AD and other dermatoses, by searching the PubMed database for studies published before October 15th, 2024. The keywords employed for the database search were “Abrocitinib.” Each article was then reviewed for data extraction.
RESULTS AND DISCUSSION
A database search showed 307 articles with the keyword abrocitinib. On excluding publications that had abrocitinib used for the treatment of AD, 37 articles remained and they were included in the review. The chosen articles were then analyzed to assess the use of abrocitinib in dermatological conditions other than AD, which gave us an outline of its off-label applications for sake of completion a synopsis of role of abrocitinib in AD was also prepared.
Mechanism of action
Abrocitinib is a selective JAK 1 inhibitor. It inhibits JAK1 binding by blocking the ATP binding site. Abrocitinib is selective for JAK1 over JAK2 (28-fold), JAK 3 (>340-fold), and tyrosine kinase 2 (43-fold). In vitro, the parent compound and its active metabolite show similar selectivity levels for JAK 1. JAK 1/2/3/TyK 2 is expressed in multiple cell types. JAK3 is commonly seen in hematopoietic cells. Various domains facilitate the binding of JAKs to intracellular receptors. Pseudokinase domains play a regulatory role.
Step 1: When the ligands bind to the cell receptors, there is dimerization which brings the associated JAKs close together. The JAK phosphorylates each other on tyrosine residues and then increases the activity of kinase domains. Then, they phosphorylate the tyrosine residues on the receptor subunit.
Step 2: Once phosphorylation occurs, it creates a binding site for the SH2 domains of the intracytoplasmic transcription factors. This is known as STAT
Step 3: The STATs are phosphorylated by JAKs which form hetero/homodimers and the dimer translocates into the nucleus which regulates the target gene expression of inflammatory mediators.
JAK-STAT is the common signal transduction pathway of cellular proliferation, migration, differentiation, and apoptosis [Figure 3].[4]
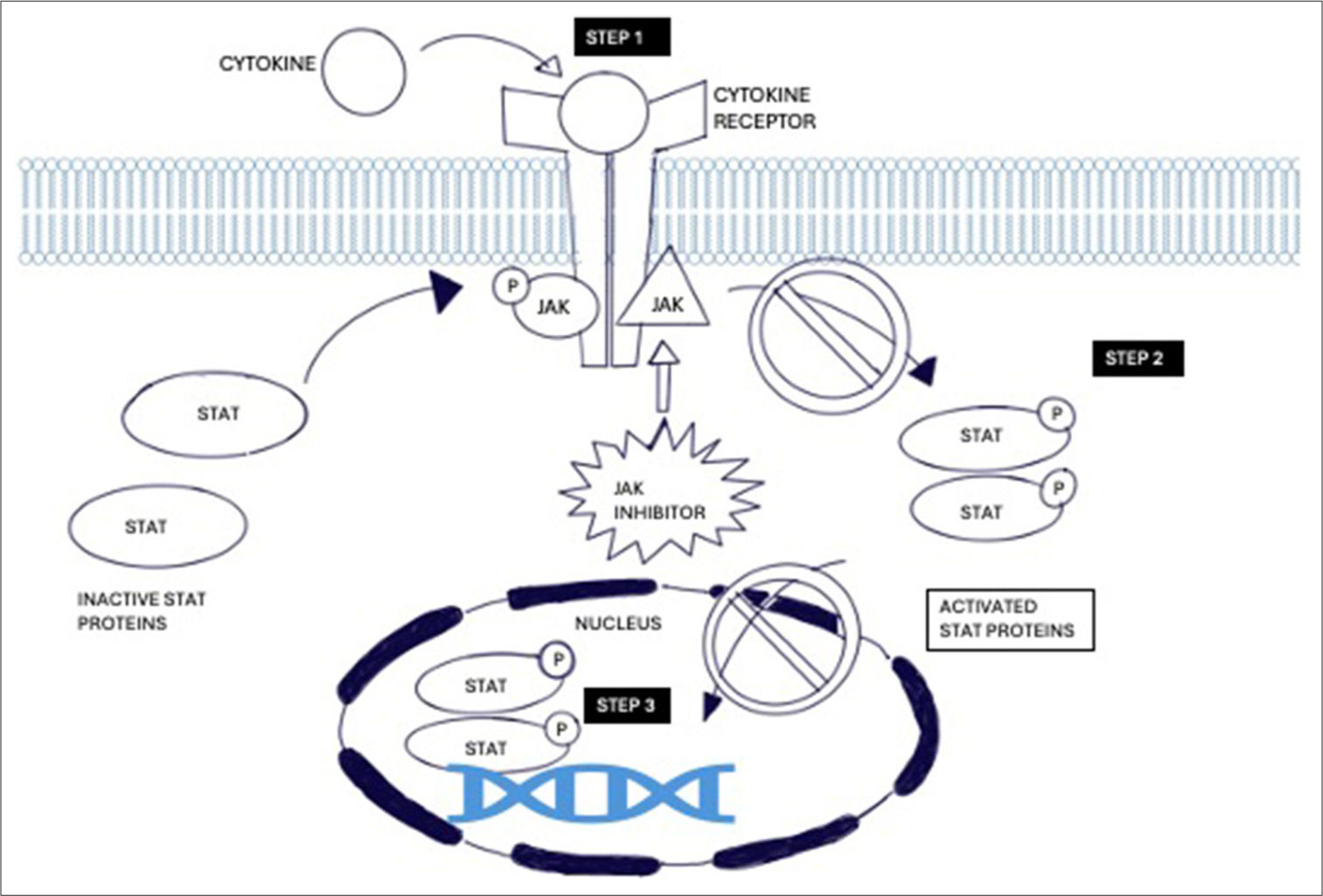
- Mechanism of action of Janus kinase signal transducers and activators transcription inhibitors in cytokine mediation. JAK: Janus Kinase, STAT: Signal transducers and activators of transcription
Pharmacokinetics
Abrocitinib has 91% oral bioavailability. Systemic drug absorption is not influenced by food. Peak concentrations of the drug are reached within 1 hour of drug intake. The t ½ of the drug is 5 hours.
When taken once daily, the drug reaches steady-state plasma concentrations within 48 hours.
The following are the drug metabolites of abrocitinib –
Pyrrolidinone pyrimidine – inactive
2-hydroxypropyl – active
3-hydroxypropyl – active.
The drug undergoes enzymatic metabolism through mostly CYP2C19 (53%), and CYP2C (30%), and a small percentage of the drug is metabolized by CYP3A4 and CYP2B6. When administered with strong CYP2C19 inhibitors, the dose of the drug should be reduced by half. When administered by CYP2C119/2C9 inducers, no dose adjustment is needed.[4] Patient characteristics including age, weight, sex, race, and CYP2C19/CYP2C9 genotype have no apparent effect on the metabolism of the drug. In adolescent patients, no difference in metabolism has been seen as compared to adult patients. There is no clinically significant difference in the active moiety of the drug in mild or moderate hepatic impairment or in mild renal impairment.
It is contraindicated in patients with severe hepatic impairment, considering the absence of studies in this population.
It has not been evaluated in patients with end-stage renal disease or renal replacement therapy.[3]
Pharmacodynamics
Reduction in JAK1 activity reduces downstream thrombopoietin production which consequently shows a fall in platelet count peaking at 4 weeks of treatment. The platelet count gradually rises back up starting at 4 weeks of treatment. After 4 weeks of abrocitinib treatment, there is also a dose-dependent increase in low-density lipoprotein (LDL) cholesterol, high-density lipoprotein cholesterol, and an increase in total cholesterol levels. A 10% rise in LDL cholesterol is seen with 100 mg of abrocitinib and a 15% rise is seen with 200 mg of abrocitinib.[4,5]
ABROCITINIB IN ATOPIC DERMATITIS
Abrocitnib has been approved for the treatment of moderate-to-severe AD in adults and adolescents of 12 years and older in patients who are candidates for systemic therapy. Approval status has been given for use in the UK, Japan, European Union, and US-FDA.
The only approved indication of abrocitinib use is AD, with oral and topical JAK inhibitors gaining rapid leverage in the treatment of AD. Second-generation JAK inhibitors act against a single isoform of JAK. This selective inhibition sidesteps the undesirable effects of JAK2 inhibition such as neutropenia and anemia. Multiple studies were done to assess the effectiveness of abrocitinib in AD and its safety in different age groups and to compare it to pre-existing treatment agents like dupilumab. Comprehensive details of abrocitinib usage in AD are covered in Table 1 and details are as follows:
| No. | Author | Study design | Sample size | Intervention | Outcome | Result |
|---|---|---|---|---|---|---|
| 1 | Simpson et al. (July 2020)[6] | A multicenter, double-blind, randomized phase 3 trial (JADE MONO -1), patients (aged≥12 years) with moderate-to-severe AD with a body weight of 40 kg or more (JADE MONO-1). | 387 | 387 participants with moderate to severe AD were selected and randomly assigned to 200 mg abrocitinib, 100 mg abrocitinib, or placebo in a 2:2:1 ratio. 154 participants were assigned to 200 mg abrocitinib group, 156–100 mg abrocitinib group, and 77 were assigned to placebo. | The primary endpoint of study was 75% improvement in symptoms after 12 weeks. | 40% of the 100 mg group saw at least a 75% improvement in symptoms with 63% of the 200 mg group reaching the same endpoint. 12% of the placebo group also saw a 75% improvement in symptoms over 12 weeks. |
| 2 | Silverberg et al. (October 2020)[7] | A phase 3, double-blinded, placebo-controlled, parallel-group randomized clinical trial included patients 12 years or older with a clinical diagnosis of moderate-to-severe AD for at least 1 year and inadequate response to topical medications given for at least 4 weeks within 6 months. | 391 | 155 participants were assigned to the 200 mg abrocitinib group, 158 to the 100 mg abrocitinib group, and 78 were assigned to the placebo group. | The primary endpoint of the study was a 75% improvement in symptoms after 12 weeks. | In the 12-week trial, 44.5% of the 100 mg group and 61% of the 200 mg group achieved at least a 75% improvement in symptoms, compared to 10.4% in the placebo group. More patients in the placebo group also discontinued treatment. |
| 3 | Blauvelt et al. (May 2021)[8] | A multicenter, double-blind, randomized phase 3 trial (JADE REGIMEN), patients (aged ≥12 years) with moderate-to-severe AD with a bodyweight of 40 kg or more. (JADE REGIMEN) | 798 | Patients received 200 mg of abrocitinib daily for 12 weeks. Those achieving clear or nearly clear skin entered a 40-week phase, where they were randomized (1:1:1) to continue 200 mg (n=266), switch to 100 mg (n=265), or receive a placebo (n=267). Patients experiencing AD flares during this period entered a rescue phase with 200 mg of abrocitinib and topical treatment to restore disease control. | The primary endpoint was the need for rescue medication due to loss of response during the maintenance phase. | During the rescue period, flares occurred in 16.2% of the 200 mg group, 39.2% of the 100 mg group, and 76.4% of the placebo group. Response was regained with 200 mg of abrocitinib and topical treatments in 36.6%, 58.8%, and 81.6% of these groups, respectively, highlighting the effectiveness of 200 mg abrocitinib in restoring response after relapse. |
| 4 | Eichenfield et al. (June 2021)[9] | A randomized clinical trial, phase 3, randomized, double-blind, placebo-controlled study was conducted in patients aged 12–17 years with moderate-to-severe AD and an inadequate response to topical medication or requiring systemic therapy. | 287 | Participants with moderate-to-severe AD were randomly assigned to receive either 200 mg of abrocitinib, 100 mg of abrocitinib, or a placebo in a 1:1:1 ratio. | The primary endpoint of the study was achieving clear or almost clear skin and/or a 75% improvement in symptoms after 12 weeks. | Over 12 weeks, 41.6% of the 100 mg group and 46.2% of the 200 mg group achieved at least a 75% improvement in symptoms, compared to 24.5% in the placebo group |
| 5 | Bieber et al. (October 2021)[10] | Phase 3, multicenter, randomized, double-blind, placebo-controlled trial unresponsive to topical therapy or needed systemic therapy -with secondary objective of comparison of efficacy with Dupilumab on the basis of reduction of itch JADE COMPARE trial. | 838 | Patients were randomly assigned to be administered 200 mg or 100 mg daily oral dose of abrocitinib, 300 mg of dupliumab, which is a competing therapy, subcutaneously every other week, or a placebo for a period of 16 weeks in a 2:2:2:1 ratio | A reduction in the severity of AD to clear or mild after 16 weeks of treatment. | Reduction in severity of AD was seen in 48.4% of the 200 mg abrocitinib group, 36.6% of the 100 mg abrocitinib group, 36.5% of the 300 mg Dupilumab group, and 14% of the placebo group by week 12. A greater reduction in itch was seen with 200 mg oral abrocitinib at week 2 as compared to Dupilumab. |
| 6 | Reich et al. (July 2022)[12] | Efficacy and safety of abrocitinib vs Dupilumab in moderate to severe AD - a randomized, double-blind, multicenter phase 3 trial. Randomized, double-blind, active-controlled, parallel-treatment (JADE COMPARE). | 727 | The abrocitinib group was given 200 mg of abrocitinib orally daily while the dupilumab group was given 300 mg of dupilumab every 2 weeks, after a 600 mg loading dose, subcutaneously. | Reduction in severity from moderate to mild or clear AD. | 48% of the abrocitinib group reached the primary endpoint by week 2 of the study while only 26% of the dupilumab group reached the primary endpoint in the same amount of time. At the end of the 26-week trial, 68% of the abrocitinib group reached the primary endpoint while 63% of the dupilumab group reached the primary endpoint. |
| 7 | Shi et al. (August 2022)[11] | Efficacy and safety of abrocitinib in adults with moderate-to-severe AD after switching from dupilumab (JADE EXTEND) -Patients with moderate-to-severe AD received abrocitinib 200 mg or 100 mg once daily in JADE EXTEND (phase 3 extension) after dupilumab in double-blind, placebo-controlled phase 3 JADE COMPARE. | 203 | Focused on the dupilumab-treated patient population from JADE COMPARE and 203 patients from the dupilumab group were used in the study. 73 were given 200 mg of abrocitinib orally and the other 130 were given 100 mg of abrocitinib orally. | Reduction in severity from moderate to mild or clear AD. | Patients who responded to prior dupilumab in JADE COMPARE maintained most of these clinical benefits with abrocitinib treatment for 12 weeks in JADE EXTEND. Additionally, large proportions of patients not responsive to dupilumab in JADE COMPARE achieved clinical benefit with abrocitinib treatment for 12 weeks, with clearance of dermatitis and itch relief. |
| 8 | Reich et al. (February 2023)[12] | Phase 3, long-term extension study with patients from previous abrocitinib AD trials, to evaluate the abrocitinib efficacy up to 48 weeks and long-term safety in patients with moderate- to-severe AD. This analysis includes data from adolescent (12 to <18 years of age) and adult (≥18 years of age) patients who received prior treatment with abrocitinib in JADE MONO-1, JADE MONO-2 and JADE COMPARE. | 1116 | Patients who had previously participated in JADE MONO-1, JADE MONO-2 or JADE COMPARE, were continued and the dose in JADE EXTEND remained consistent with the qualifying study, in the 200 mg or 100 mg groups. | To evaluate the abrocitinib efficacy up to 48 weeks and long-term safety in patients with moderate-to-severe AD. | About 70% and 45% of patients received a≥48 weeks, respectively. At week 48, almost to complete clearance was seen in 52% patients receiving 200 mg abrocitinib and 39% patients on 100 mg abrocitinib. Significant improvement in skin lesions and pruritus was seen in moderate to severe AD. |
AD: Atopic dermatitis, JADE: JAK1 atopic dermatitis efficacy and safety
JADE MONO-1, Simpson et al. (July 2020)
A multicenter, double-blind, placebo-controlled, phase 3 trial was conducted in patients with moderate-to-severe AD of age 12 years or above, with a body weight of 40 kg or more. Both 200 mg and 100 mg doses of abrocitinib demonstrated significant efficacy in improving the signs and symptoms of moderate-to-severe AD compared to placebo. By week 12, a significantly greater proportion of patients in the abrocitinib 100 mg and 200 mg groups achieved an Investigator Global Assessment response and at least a 75% improvement in their Eczema Area and Severity Index scores compared to the placebo group. Notable reductions in pruritus were observed as early as the first post-baseline assessment. The 12-week study highlighted a favorable safety profile for abrocitinib, with no reported cases of venous thromboembolism, malignancy, major adverse cardiovascular events, or deaths.[6]
Silverberg et al. (October 2020)
This phase 3, double-blind, placebo-controlled, parallel-group randomized clinical trial involved patients aged 12 years and older who had moderate-to-severe AD for at least 1 year and an inadequate response to topical therapies administered for a minimum of 4 weeks within the past 6 months. The findings demonstrated that abrocitinib is an effective treatment for AD, with a low incidence of treatment-related adverse effects, making it a promising and safe therapeutic option.[7]
JADE REGIMEN, Blauvelt et al. (May 2021)
A phase 3 trial investigated the effects of randomized withdrawal of abrocitinib in patients undergoing treatment. This study assessed the necessity of maintaining a consistent dose and the flexibility of the treatment regimen. Patients initially received 200 mg of abrocitinib daily for 12 weeks. Those achieving clear or nearly clear skin then entered a 40-week phase, during which their dose was adjusted to either 200 mg, 100 mg, or a placebo. Patients who experienced an AD flare during this period entered a rescue phase with 200 mg of abrocitinib and a topical treatment to regain disease control. A high rate of relapse was seen with treatment discontinuation but the treatment response seen previously was recaptured with 200 mg of abrocitinib.[8]
JADE TEEN, Eichenfield et al. (June 2021)
A phase 3 trial was conducted to understand the safety and efficacy of oral abrocitinib in patients between 12 and 17 years of age, with moderate-to-severe AD, not responsive to topical therapy. At week 12, 41.6% in the 100 mg group and 46.2% in the 200 mg group achieved over 75% clearance in dermatitis. 2.1% in the 200 mg group had a serious adverse effect and none in the 100 mg group. The study confirmed that abrocitinib is an effective treatment for AD in adolescents, with a very low incidence of serious adverse effects, consistent with previous results.[9]
JADE COMPARE, Bieber et al. (October 2021)
The study was a multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of two abrocitinib doses in adults with moderate-to-severe AD. Patients were allowed to use more than one topical therapy (low-to-medium potency topical glucocorticoids, topical calcineurin inhibitors, and topical phosphodiesterase 4 inhibitors). Patients 18 years of age or older with at least a 1-year history of AD were included.
The secondary objective was to evaluate and compare the efficacy of abrocitinib, with dupilumab based on reduction in itch at 2 weeks.
Abrocitinib 200 mg or 100 mg once daily had significantly greater reductions in moderate-to-severe atopic compared to placebo at weeks 12 and 16. The 200-mg dose of abrocitinib was superior to dupilumab with respect to relief of pruritus at week 2.
More adverse events were seen with Abrocitinib (Ab) 200 mg dose, compared to the lesser dose. 100 mg of Ab had adverse effects comparable to dupilumab.
The main adverse events with abrocitinib were nausea, acne, nasopharyngitis, and headache.[10]
JADE EXTEND, Shi et al. (December 2022)
An extended phase 3 study was conducted to assess the effectiveness of abrocitinib in patients who had earlier been treated with dupilumab. After the 12-week study, 76.9% of patients in the 100 mg abrocitinib group who had previously achieved the endpoint with dupilumab also met the endpoint with abrocitinib. Similarly, 83.3% of those in the 200 mg abrocitinib group who initially achieved the endpoint with dupilumab maintained their response. Among patients who did not respond to the initial dupilumab trial, 35.2% in the 100 mg abrocitinib group and 47.2% in the 200 mg group successfully reached the endpoint. These findings demonstrate that abrocitinib is effective in nearly half of the cases where dupilumab was previously ineffective. This could be attributed to abrocitinib’s ability to target cytokines that are more directly involved in the pathophysiology of AD compared to dupilumab.[11]
JADE EXTEND, Reich et al. (February 2023)
This Phase 3 long-term extension study enrolled patients who had participated in previous abrocitinib trials for AD providing long-term safety information for abrocitinib in a population with a median drug exposure of 10.4 months, where approximately 45% of patients had been exposed to the drug for ≥48 weeks. The most frequent adverse events included nasopharyngitis, AD, nausea, upper respiratory tract infections, acne, increased creatine phosphokinase, and herpes simplex.[12]
Long-term efficacy data showed:
Sustained control of AD was seen with abrocitinib without the need for concomitant topical medications.
A significant proportion of patients who did not meet formal response criteria by week 12 showed clinically meaningful improvements by week 24.
Serious adverse effects occurred in 7% of patients receiving abrocitinib 200 mg and 5% of those on 100 mg, with study discontinuation in 9% and 7% of patients receiving 200 mg and 100 mg, respectively.
ABROCITINIB IN OTHER DERMATOSES
However, considering the role of JAK inhibitors in other inflammatory and autoimmune dermatoses, this review examines other published data where abrocitinib has been used in the treatment of other dermatoses, which are frequently refractory to other treatments as reported. Demonstration of therapeutic use has mostly been restricted to isolated case reports and small groups of patients [Table 2].
| Condition | Level of evidence |
|---|---|
| Eczema | |
| Moderate to severe AD in adults.* | Level 2 |
| Occupational airborne allergic contact dermatitis (ABCD) | Level 4 |
| Nipple and areola eczema | Level 4 |
| Hand eczema | Level 4 |
| Autoimmune conditions | |
| AA | Level 4 |
| LS | Level 4 |
| PG | Level 4 |
| LV | Level 4 |
| Oral LP | Level 4 |
| Vitiligo | Level 4 |
| Inflammatory conditions | |
| HS | Level 4 |
| Prurigo nodularis | Level 4 |
| Necrobiosis lipoidica | Level 4 |
| Lichenoid amyloidosis | Level 4 |
| Disorders of keratinization | |
| PPK | Level 4 |
| HHD | Level 4 |
| Netherton syndrome | Level 4 |
| Others | |
| Pruritus of unknown origin | Level 4 |
Alopecia areata (AA)
In AA, there is a T-cell-mediated autoimmune destruction of hair follicles with resultant nonscarring patchy hair loss. The extent of involvement varies in the patient and may be restricted to the scalp or involve other hair-bearing areas. The condition carries significant detriment to the patient’s psychological health.
Tofacitinib, a JAK 1 and JAK 3 inhibitor, has been increasingly popular in the treatment of AA. Several case reports and case series have reported successful treatment of AA and alopecia universalis with abrocitinib at 100 mg daily and 200 mg daily doses.
In comparison to other dermatological conditions, a long median time to complete resolution (about 10 months) was noted in AA.[13-15]
Lichen sclerosus (LS)
LS is a non-specific inflammatory genital dermatosis, and a key regulator is interleukin (IL)-6 which has a pro-inflammatory role. It has been hypothesized that LS has more of an autoimmune disease with a preferable Th1 immune response and increased expression of proinflammatory cytokines specific to Th1-interferon (IFN) gamma-induced immune response. Abrocitinib, as an inflammatory agent, also is considered to reduce the release of IL-6.
A demonstrable effective response was seen in a 50-year-old male with plasma cell balanitis and LS where there was complete remission within 1 month of abrocitinib 100 mg daily.[16]
Abrocitinib was also used in 10 patients, 7 females, and 3 males, mean age 35.4 years, who had been treated with different topical agents for LS and had an inadequate response. They were given a 100 mg daily dose of abrocitinib, which was continued for 4 months after all patients showed good outcomes. One patient developed dyslipidemia and had a fall in their platelet count at the end of the treatment period. The rest showed no adverse effects.[17]
Pyoderma gangrenosum (PG)
Abrocitinib has been effectively used in a 16-year-old male with perianal PG. After worsening symptoms with doxycycline, isotretinoin, oral steroids, and cyclosporine A, the patient was started on abrocitinib 100 mg daily. Improvement was seen within a week, and within a month, the ulcer size reduced significantly, with alleviation of swelling and discharge. During the 4-month follow-up period, further improvement was maintained.[18]
Livedoid vasculopathy (LV)
In a 31-year-old female with LV for over 2 years, when adequate response was not seen with glucocorticoids, thalidomide, hydroxychloroquine, doxycycline, or cyclosporine, the patient was started on 100 mg abrocitinib. There was rapid improvement in pain, swelling, and ulceration with complete remission in 6 weeks.
During the next 12-week follow-up period, when the dose was tapered to 100 mg once every 2 days, there was no recurrence. No adverse effect was noted.[18]
Hidradenitis suppuritiva (HS)
In a 17-year-old male, with axillary HS with recurrent inflammatory lesions over the 3-year duration, minimal resolution was seen despite prolonged medical and surgical treatment. Following such a poor clinical outcome, the patient was started on abrocitinib at 100 mg once daily.
With treatment, there was a significant reduction in the pain and size of abscesses. Excellent improvement was seen in 2 weeks, and the drug was continued for 6 weeks, then reduced to a dose of 100 mg every 2 days. No lesions were reported during a follow-up period of 10 weeks.[18]
Lichenoid amyloidosis
Significant lesional improvement with symptomatic betterment was seen in 2 patients (53-year-old female and 59-year-old female) with daily abrocitinib 100 mg on treatment for 2–4 months, and sustained improvement was seen when the drug was tapered to once in 2–3 days.[19]
Porokeratosis
Abrocitinib has been effective in the treatment of eruptive pruritic papular porokeratosis with rapid resolution of pruritus. Increased IL-31 cells are seen in the lesional skin. Dysregulated keratinocyte proliferation also causes a Th2 inflammatory response which further upregulated IL-31. Abrocitinib through JAK1 blockage inhibits IL-31 by over 85%. In a 75-year-old male with porokeratosis for 60 years, rapid relief in itching was seen in 24 hours followed by subsidence of skin lesions within 30 days of treatment.[20]
Hailey–Hailey disease (HHD)
HHD is a rare genodermatosis with a mutation in the ATP2Cl gene that causes impaired Ca+2 transport signaling. It presents as painful erythematous, blistering skin at sites of friction, that will then erode with crusting, scaling, and hypertrophic vegetative growths. The primary skin barrier defects in HHD lead to cytokine-mediated secondary Th2 inflammation. The use of JAK inhibitors inhibits the Th2- mediated IL-4 and IL-13 signaling, which also helps in Ca+2 mobilization in keratinocytes.[21]
A 41-year-old male with HHD was successfully treated with 4 weeks of abrocitinib 100 mg daily.[22]
Necrobiosis lipoidica
A 53-year-old female with extensive NBL for over 10 years, treated previously with steroids, calcineurin inhibitors, psoralens, and hydroxychloroquine, was treated with oral abrocitinib. Treatment was started with 200 mg abrocitinib once daily for 11 months.[23]
Vitiligo
Vitiligo is due to the autoimmune destruction of melanocytes by self-reactive CD8+ T lymphocytes. CD8+ T cells produce IFN gamma which then causes JAK-STAT phosphorylation. JAK inhibitors, both in topical form (ruxolitinib 1.5%, tofacitinib 2%, ifidancitinib 0.46%, delgocitinib) and oral form (tofacitinib, baricitinib), have proven to be effective in treatment.[1]
A 61-year-old male with active acrofacial vitiligo, inadequate response to topical tacrolimus 0.1%, was treated with 100 mg daily abrocitinib and had significant repigmentation. After 2 months, there was no recurrence of the patches, and was successfully maintained on topical Tacrolimus, with repigmentation developing 4 months after abrocitinib stoppage.[24,25]
In a case series, Xu et al. treated 11 patients of refractory progressive vitiligo, between 26 and 59 years of age (mean age 35.9 years, mean disease duration 17.7 years) – 2 males and 9 females – with Abrocitinib 100 mg daily for 16 weeks. Favorable clinical outcomes were seen in all, with disease stability achieved. 10 patients continued treatment with 100 mg every alternate day for 2 months, along with narrow-band ultraviolet B therapy. Headache, dizziness, and nausea were noted in 3 patients.[26]
Oral lichen planus (LP)
LP is an inflammatory condition with T-cell-predominant lymphocytic reactions. Reticulated white patches are seen over the oral mucosa associated with burning or increased gustatory sensitivity in oral LP.
JAK-STAT cytokines have been proposed to play a definitive role in disease pathogenesis. JAK inhibitors have demonstrated effectiveness in the treatment of oral LP, including tofacitinib, baricitinib, and upadacitinib.[26]
A 58-year-old male, who had failed to show effective response to other immunosuppressants, responded well to abrocitinib 200 mg once daily for 7 days, followed by 100 mg daily, with improvement in 3 months.[27]
Allergic contact dermatitis (ACD)
ACD is a delayed type IV hypersensitivity reaction where diffuse eczematous lesions manifest within hours to days after exposure to a contact allergen.[28] Complete clearance was reported in a 37-year-old male with ACD to Compositae species, where 100 mg abrocitinib was given daily for 8 weeks.[29]
Nipple and areola eczema
Nipple eczema is seen in patients with AD, with a pathogenic role of the same Th2 inflammatory pathways. A 28-year-old male, recently recovered from severe acute respiratory syndrome coronavirus 2 infection, with background atopy, presented with complaints of nipple eczema. Dermatitis had limited response to emollients, steroids, calcineurin inhibitors, and phototherapy. He was started on 100 mg abrocitinib once daily and pruritus reduced remarkably within 24 hours. Treatment was continued for 12 weeks and the lesions completely cleared. No relapse was seen in 3 months after treatment cessation.[30]
Prurigo nodularis (PN)
PN occurs with hyperplasia of intraepidermal neuronal fibers with proinflammatory cytokine release such as IL-4, IL-17, IL-22, and IL-31. An increase in expression of STAT 3/6 has been seen in lesional biopsies which underline the therapeutic role of JAK-STAT inhibitors. Tofacitinib and baricitinib have been demonstrated to be useful in the treatment of PN.[31]
A 62-year-old female, with a 2-year history of PN, had complete resolution with 2 months of 100 mg abrocitinib and topical ruxolitinib, triamcinolone, and crisaborole. Unsatisfactory responses had been seen with dupilumab.[32]
Hand eczema
Eczema of the hands is a chronic and recurrent dermatoses that significantly affect the quality of life. JAK inhibitors are considered beneficial in the treatment of hand eczema. In a case series of 12 patients, 7 males and 5 females, mean age 46.3 ± 14.1 years, abrocitinib 100 mg once daily was administered and significant improvement was seen at week 16, with over 90% clearance in the lesions. 7/12 patients experienced adverse effects including nausea, headache, dizziness, and blurred vision – none of which required stoppage of the drug.[33] Other similar instances of abrocitinib use include a case of refractory hand and feet eczema treated successfully,[34] and a series of 17 patients, of which 13, showed over 90% clearance by week 16.[35]
Pruritus of unknown origin
Chronic pruritus of unknown origin or CPUO is a condition where there is persistent itching for over 6 weeks, without any identifiable cause. It involves both local and systemic type 2 inflammation which targets the type 2 cytokines (IL-4 and IL-13) thereby causing itch sensation over the sensory neurons. Dupilumab has been used to treat CPUO successfully. Kwatra et al. reported a case series wherein 10 patients with CPUO were treated with 200 mg abrocitinib once daily for 12 weeks, and all had favorable clinical outcomes. However, 2/10 patients developed mild adverse effects including scalp folliculitis, acneform eruptions, and herpes labialis.[35]
Netherton syndrome
Netherton syndrome is a genodermatosis involving the skin, hair, and immune system. An underlying increase in serum immunoglobulin E levels and hypereosinophilia is seen, which is similar to AD. It is regulated by activated kallikreins which are serine proteases that cause overexpression of proallergic and proinflammatory cytokines. Persistent increased levels of kallikrein 5 induce an overexpression of proinflammatory Th2 and Th17 activity.[36,37]
A 28-year-old female with Netherton syndrome was treated with dupilumab but had a recurrence of lesions. She was switched to abrocitinib, and there was rapid and complete clearance in 3 weeks with 200 mg daily for 1 week. Since the patient complained of nausea and lightheadedness, it was tapered to 100 mg daily.[38]
Previous treatment included steroids, methotrexate, cyclosporine, and secukinumab.
Table 3 gives an overview of the various dermatoses in which oral abrocitinib has been used with the clinical outcome and observed adverse effects.[39-55]
| No. | Dermatological Indication | Type of Study | Patient Population | Dose and duration of Abrocitinib | Concomitant treatment. | Results | Adverse Effects | Author |
|---|---|---|---|---|---|---|---|---|
| 1 | Alopecia Areata after DRESS | Case Report | 30 y/Female | 100 mg OD x 2 months, then 200 mg OD for 2 months, then tapered down to 100 mg OD for 6 months |
None | Significant improvement | None | Zhang et. al.[14] |
| Case Report | 40y/Female | 100 mg OD | None | Improved at 1 week, rebound at 8 weeks stopping, good results at 12 weeks. |
None | Cai L et. al.[51] | ||
| 2 | Pediatric Alopecia Areata | Case Report | 11 y/Male | 100 mg OD for 4 months | None | Hair regrowth on the scalp | None | Huang et. al.[13] |
| 3 | Alopecia Areata with Atopic Dermatitis | Case Report | 14 y/Female | 200 mg OD for 3 months | None | Complete regrowth of the hair with subsidence of AD lesions | None | Zhao J et. al.[15] |
| Case Report | 13 y/Male | 100 mg OD for 2 months followed by 200 mg OD for 2 months then reduced to 100 mg once daily for 6 months. | None | Significant hair regrowth was seen at 6 months | None | |||
| 4 | Lichen Sclerosus | Case Series | 7 females and 3 males between 22 to 48 years of age |
100 mg OD | None | Disease control seen in 3 months |
None | Bao et. al.[16] |
| 5 | Lichenoid Amyloidosis | Case Report | 1 male and 1 female | 100 mg once daily for 2-4 months, tapered to once in 2-3 days |
None | Marked improvement in 8 weeks | None | Bai et. al.[19] |
| 6 | Hailey Hailey Disease | Case Report | 41 y/Male | 100 mg OD | Topical zinc oxide | Evident clinical improvement after 4 weeks | None | Mitroi et. al.[23] |
| 7 | Necrobiosis Lipoidica | Case Report | 53 y/Female | 200 mg OD x 11 weeks, then 100 mg OD | NR | Improvement after 11 months | Mild abdominal pain | Arnet et. al.[24] |
| 8 | Allergic Contact Dermatitis - occupational airborne | Case Report | 37 y/Male | 100 mg OD | None | Marked clearance in 2 months |
None | Baltazar et. al.[30] |
| 9 | Pyoderma Gangrenosum | Case Report | 16 y/Male | 100 mg OD | Cyclosporine 50 mg BD for 3 weeks | Significant improvement after 4 weeks | None | Chen et. al.[18] |
| 10 | Livedoid Vasculopathy | Case Report | 31 y/Female | 100 mg OD x 4 weeks, followed by 100 mg every 2 days. | None | Complete remission after 6 weeks |
None | Chen et. al.[18] |
| 11 | Hidradenitis Suppuritiva | Case Report | 17 y/Male | 100 mg OD x 4 weeks, followed by 100 mg every 2 days. | Doxycycline 100 mg BD for 2 weeks | Almost complete clearance after 6 weeks |
None | Chen et. al.[18] |
| 12 | Netherton Syndrome | Case Report | 28 y/Female | 200 mg OD x 1 week, tapered to 100 mg OD x 3 weeks | None | Significant improvement after 3 weeks | Light-headedness, nausea | Zheng et. al.[39] |
| 13 | Oral Lichen Planus | Case Report | 58y/Male | 200 mg OD x 12 weeks, tapered to 100 mg once daily | None | Good response after 12 weeks | None | Solimani et. al.[28] |
| 14 | Nipple-Areola Eczema | Case Report | 28 y/Male | 100 mg OD x 12 weeks | None | Marked improvement in 12 weeks and no relapse at 20 weeks | None | Teng et. al.[31] |
| 15 | Vitiligo - Progressive | Case Series | 11 patients - 2 Males and 9 Females - Mean Age 35.9 years | 100 mg OD x 16 weeks tapered to 100 mg every 2 days | Phototherapy | Favourable outcomes | Headache, dizziness, nausea, abdominal discomfort (27.27%) | Xu Z et. al.[26] |
| 16 | Vitiligo - Acrofacial Progressive | Case Report | 61 y/Male | 100 mg OD | None | Significant repigmentation and no progression at 2 months follow up, was restarted on topical tacrolimus 0.1% daily | None | Satkunathan et. al.[25] |
| 17 | Hand Eczema | Case Series | 12 patients - 7 Males and 5 Females, Mean Age 46.3 y | 100 mg OD | None | Significant improvement at 16 weeks follow up | Nausea (58%), headache, acne, dizziness, blurred vision in others. | Li Y et. al.[36] |
| 18 | Chronic Pruritus of Unknown Origin | Case Series | 10 patients - 8 Males and 2 Females, Mean Age 70.7 y | 200 mg OD x 12 weeks | None | Significant improvement at 12 weeks; treatment discontinued thereafter. | Scalp folliculitis, acneiform eruption, herpes labialis | Kwatra SG et. al.[35] |
| 19 | Pityriasis Rubra Pilaris | Case Series | 5 patients - 2 Males and 3 Females, Mean Age 47.7 y | 100 mg OD | None | At 4 weeks, PASI 24.6 ± 19.61 became 4.6 ± 4.03, and BSA of 39.6 ± 33.41 became 13.6 ± 11.89. |
None | Li Y et. al.[40] |
| 20 | Erythematotelangiectatic Rosacea | Case Series | 4 females, Mean age = 37.5 y | 100 mg OD for 16 weeks followed by once every 4 days | Significant improvement after 4 weeks in 1 patient, Mild improvement in 2 patients, and no improvement in 1 patient. | None were reported, and 1 patient was discontinued due to Hepatitis B detection. | Zhang T et. al.[41] |
|
| 21 | Granulomatous Rosacea | Case Report | 53 y/Female | 100 mg OD for 20 weeks followed by 100 mg once weekly | None | Significant improvement at 20 weeks follow-up | None | Ren M et. al.[42] |
| 22 | Steroid induced Rosacea | Case Series | 4 Females, Mean Age = 40 y. | 100 mg OD | Topical azelaic acid and a skin barrier protection | Significant improvement. | None | Xu B et. al.[43] |
| 23 | Chronic Actinic Dermatitis | Case Report | 70 y/Male | 100 mg OD for 6 weeks followed by twice a week. | None | Significant improvement after 6 weeks. | None | Jin X et. al.[44] |
| 24 | Bullous Pemphigoid | Case Report | 52 y/Female, | 100 mg OD | 60 mg Methylprednisolone reduced to 2 mg daily after 1 month | Significant improvement | None | Jiang W et. al.[45] |
| Case Report | 83 y/Male | 100 mg OD | 4 mg Methylprednisolone | Significant improvement | None | Jiang W et. al.[45] |
||
| 25 | Tattoo granuloma with uveitis | Case Report | 41 y/Male | 100 mg OD | Prednisone 30 mg daily with pranoprofen eye drops for uveitis | Significant improvement with no new lesions at 6 weeks follow-up | None | Yang Y et. al.[46] |
| 26 | Granuloma annulare | Case Report | 29y/Female | 150 mg OD | NR | Significant improvement after 6 weeks | None | Liu W et. al.[47] |
| Case Report | 77y/Female | 200 mg OD | NR | Complete clearance in 3 months | Nausea, herpes labialis | Michels A et. al.[48] |
||
| 27 | Psoriasis | Case Report | 69y/Female | 100 mg OD interrupted at 12 weeks | None | Marked remission of skin lesions | None | Mao J et. al.[49] |
| 28 | Post-zygomatic arch hyaluronic acid filler: cheek and jawline edema at 6 weeks | Case Report | 55y/Female | 100 mg OD | None | Complete clearance in 2 months | None | Lopez MHP et. al.[50] |
| 29 | Eosinophilic pustular folliculitis | Case Report | 50y/Male | 100 mg OD | None | Improved at 4 weeks, and cleared by 6 months | None | Cai L et. al.[51] |
| 30 | Dissecting cellulitis of the scalp | Case Report | 27y/Male | 100 mg OD | NR | Improvement after 4 months | None | Jin S et. al.[52] |
| 31 | Mucous Membrane Pemphigoid | Case Report | 62y/Female | 100 mg OD for 4 weeks, followed by once in 2 days, for 2 months | NR | Significant improvement after 3 days, complete clearance in 4 weeks | None | Teng Y et. al.[53] |
| 32 | Porokeratosis ptychotropica | Case Report | 45y/Male | 100 mg OD for 4 weeks | None | Improvement after 4 weeks, with good outcome at 4 months | None | Zhang X et. al.[21] |
| 33 | Pruritic Papular Porokeratosis | Case Report | 75y/Male | 100 mg OD | None | Favorable outcome at 1 month follow-up | None | Xia J et. al.[20] |
| 34 | Perioral dermatitis | Case Report | 26y/Female | 100 mg OD | None | Favourable outcome at 1 month follow-up | None | Teng Y et. al.[54] |
| 35 | Pityriasis Rosea | Case Report | 25y/Female | 100 mg OD | NR | Significant improvement after 2 days; Complete clearance in 14 days | NR | Wu H et.al.[55] |
| 36 | Prurigo Nodularis | Case Report | 62y/Female | 100 mg OD | Ruxolitinib, Triamcinolone topical for 2 months | Complete clearance after 2 months | None | Vander Does A et. al.[33] |
| Case Report | 56y/Male | 100 mg OD | NR | Almost complete clearance after 2 months | NR | Sun F et. al.[34] | ||
| Case Series | 10 female patients, mean age 58.6y | 200 mg OD | None | Favourable clinical outcome in all cases | 4 cases of headache, nausea, acneiform eruption, sore throat, and nasal congestion | Kwatra SG et. al.[35] |
Dosage recommendations, prescribing information
Available dosage forms: 50 mg, 100 mg, 200 mg
Recommended dosage: 100 mg orally once daily.
If not responding to 100 mg, can be increased to 200 mg once daily (CIBINQO Prescribing Information).[56]
Dosage recommendations for special groups are listed in Table 4.
| Special group | Recommendation |
|---|---|
| Moderate renal impairment | 50 mg orally once daily. If not responding, increase to 100 mg once daily. |
| CYP2C19 poor metabolizer | 50 mg orally once daily. If not responding, increase to 100 mg once daily. |
| Pregnancy | Effects of Abrocitnib on pregnancy are unknown. It is advised to avoid in pregnancy. Patients are encouraged to contact a pregnancy register if they are on abrocitinib. |
| Lactation/Breastfeeding | • Not recommended. Abrocitinib may be started 1 day after the last dose (approximately 5–6 elimination half-lives). |
| Pediatric patients | • Not approved for use in under 12 years of age. • Reported to be effective in refractory AA in 11-year-old child, without any adverse effects.[6] |
| Geriatric patients (>65 years and older) | Higher percentage of patients, who are 65 years and older, have been discontinued from trials since a higher reduction in lymphocyte count (<500/mm3) and platelet count (<7500/mm3) is seen in this group. Higher incidence of herpes zoster is seen. |
| Severe hepatic impairment | Not recommended. |
| Severe renal impairment (eGFR 15–29 mL/min) | Mild renal impairment (60-89 mL/min) 100 mg daily Abrocitnib. Moderate renal impairment (30-59 mL/min) 50 mg daily Abrocitinib. Severe renal impairment (15-29 mL/min) Not recommended. |
| End-stage renal disease (eGFR<15 mL/min) |
Not recommended. |
eGFR: estimated Glomerular Filtration Rate
Baseline investigations and follow-up
Recommendations for baseline investigations and follow-up monitoring of oral abrocitinib are listed in Table 5.[2]
| Parameter | Baseline investigations | Periodic follow-up |
|---|---|---|
| CBC | Not recommended in patients with: - Platelet count <150,000/mm3 - Absolute lymphocyte count <500/mm3 - Absolute neutrophil count <1,000/mm3 - Hemoglobin <8 g/dL |
Repeat at 4 weeks-Repeat 4 weeks after dose increase |
| RFT | Not recommended in patients with eGFR <15 mL/min or severe renal impairment. | Baseline RFT to be done to rule out renal dysfunction. |
| LFT | Not recommended in severe hepatic impairment. | Baseline LFT to rule out hepatic dysfunction. |
| T evaluation | - Rule out active TB; abrocitinib not recommended in active TB. -For latent TB or high-risk patients, start preventive anti-TB therapy. - Monitor for TB signs and symptoms yearly. |
Monitor yearly for TB signs and symptoms.- Patients with high latent TB risk should have continued preventive measures. |
| Viral hepatitis screening | - Not recommended in active Hepatitis B or C. - Monitor HBV DNA levels in inactive Hepatitis B; consult hepatologist if DNA levels rise. |
Periodically monitor HBV DNA levels in inactive Hepatitis B. |
| Skin examinations | - Perform skin examinations before initiating therapy. - Assess for signs of malignancies, including NMSC. |
Repeat periodically for signs of malignancies, especially NMSC and lung cancers in current or past smokers. |
| Cardiovascular Risk (MACE) | - Use with caution in patients with cardiovascular risk factors or smoking history. - Inform patients of serious cardiovascular symptoms. |
Monitor for signs of MACE at 4 weeks and 4 weeks after dose increase. |
| Thrombosis risk | Avoid use in patients with an increased risk of thrombosis. | Monitor for signs of thrombosis periodically. |
| Blood lipid levels | Check baseline levels. | Repeat at 4 weeks and 4 weeks after dose increase. |
| Signs of infection | Baseline evaluation not explicitly mentioned but consider in context of active infections. | Monitor for signs of infection at 4 weeks and 4 weeks after dose increase. |
CBC: Complete blood count, RFT: Renal function test, LFT: Liver function test, TB: Tuberculosis, NMSC: Non-melanoma skin cancers, HBV: Hepatitis B virus, MACE: Major adverse cardiovascular events, T: Tuberculosis
Treatment discontinuation recommendations
Recommendations for discontinuing oral abrocitinib in patients are listed in Table 6.[2]
| Criteria | Action | Condition for reinitiation |
|---|---|---|
| Serious or opportunistic infection | Discontinue treatment. | Reinitiation must be carefully considered after controlling the infection. |
| Hematologic Abnormalities | Condition | |
| - Platelet Count <50,000/mm3 | Discontinue abrocitinib and follow with CBC until platelet count >100,000/mm3. | Do not initiate Abrocitinib in platelet count less than <150,000/mm3 |
| - ALC <500/mm3 | Temporarily discontinue treatment. | Restart when ALC returns above 500/mm3. |
| - ANC <1,000/mm3 | Temporarily discontinue treatment. | Restart when ANC returns above 1,000/mm3. |
| - Hb value <8 g/dL | Temporarily discontinue treatment. | Restart once Hb returns above 8 g/dL. |
CBC: Complete blood count, Hb: Hemoglobin, ALC: Absolute Leucocyte Count, ANC: Absolute Neutrophil Count
Instructions about administration
Abrocitinib is administered with or without food at approximately the same time each day.
Tablets must be swallowed whole with water. They should not be crushed, split, or chewed (CIBINQ prescribing information).[56]
Immunization
Before treatment, all age-appropriate vaccinations as recommended by current guidelines should be fulfilled. Avoid vaccination with live vaccines immediately before, during, and immediately after abrocitinib (CIBINQ prescribing information).[56]
Contraindications
Abrocitinib is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment.
Drug interactions
Abrocitinib dose should be reduced when it is co-administered with CYP2C19 inhibitors and it should be avoided with drugs that are both CYP2C19/CYP2C9 inhibitors.
Abrocitinib should be avoided with strong CYP2C19 or CYP2C9 inducers.[56]
Side effects
Adverse effects noted with oral abrocitinib include those ranging from serious infections, malignancies, cardiac events, and increased prothrombotic events, as listed in Table 7.[56]
| Side effect | Details | Recommendations |
|---|---|---|
| Serious infections | Vulnerable to serious infections, including increased risk of herpes zoster. | Counsel patients about infection signs and advise contacting healthcare providers immediately. |
| Malignancies (including skin cancers) | Increased risk of malignancies, including skin cancers. | Periodic skin examinations; counsel on wearing protective clothing and using broad-spectrum sunscreen. |
| MACE | Increased risk of MI, stroke, and other cardiovascular issues. | Baseline monitoring; counsel on smoking cessation and cardiovascular risk factors. Warn about symptoms like chest discomfort, slurred speech, and lightheadedness. |
| DVT and PE | Increased risk of DVT and PE. | Counsel on signs such as leg swelling or pain, chest or back pain, and shortness of breath. |
| Retinal detachment | Retinal detachment reported during drug trials for AD. | Advise immediate reporting of vision blurring or difficulty. |
| Live vaccines | Live vaccines are not recommended during treatment, or immediately before starting the drug. | Avoid live vaccines while taking abrocitinib. |
MACE: Major adverse cardiovascular events, DVT: Deep vein thrombosis, PE: Pulmonary embolism, AD: Atopic dermatitis, MI: Myocardial Infarction
Among AD patients, the frequency of side effects is similar with doses of 100 mg and 200 mg. Common adverse effects (AEs) in clinical practice are headache, nausea, nasopharyngitis, and acne, and these are mostly transient and mild to moderate in nature. Black box warnings for Janus Kinase inhibitors (JAKi) should prompt careful patient selection.[56]
Cost of treatment
The upfront price of abrocitinib is presumably more than other drugs for moderate-to-severe AD, but treatment with abrocitinib has shown that it saves 11000–13000 dollars annually per patient while factoring in the finances saved through reduction in doctors’ visits and improved work quality of life. Overall, this increases the cost-effectiveness of abrocitinib in moderate-to-severe AD.
CONCLUSION
This review suggests that abrocitinib holds potential as a treatment for a range of dermatological conditions beyond AD. However, it is important to recognize the limitations of the available evidence. Most of the current data come from case reports and small case series, which limits the ability to generalize findings and draw firm conclusions regarding its safety and effectiveness. Therefore, these preliminary results should be viewed with caution. To gain a more thorough understanding of abrocitinib’s potential in treating various skin conditions, future research should prioritize large-scale, randomized controlled trials. Critical areas for further investigation include assessing the long-term efficacy and safety of abrocitinib, as well as comparing it directly to established treatments. Such studies are essential to confirm these initial findings and assess the broader applicability of abrocitinib in clinical dermatology. Until such evidence becomes available, clinicians should use their clinical judgment carefully when considering the off-label use of abrocitinib, given its exploratory nature.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Deepika Pandhi is the Editor in Chief of the journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Janus-kinase inhibitors in dermatology: A review of their use in psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease. Indian J Dermatol Venereol Leprol. 2024;90:30-40.
- [CrossRef] [PubMed] [Google Scholar]
- Abrocitinib: A new FDA-approved drug for moderate-to-severe atopic dermatitis. Ann Pharmacother. 2023;57:86-98.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of JAK1 inhibitor abrocitinib in atopic dermatitis. Pharmaceutics. 2023;15:385.
- [CrossRef] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. Abrocitinib. 2012. LiverTox: Clinical and research information on drug-induced liver injury. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548976 [Last accessed on 2022 Jun 13]
- [Google Scholar]
- Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet (London, England). 2020;396:255-66.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156:863-73.
- [CrossRef] [PubMed] [Google Scholar]
- Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 atopic dermatitis efficacy and safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86:104-12.
- [CrossRef] [Google Scholar]
- Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: The JADE teen randomized clinical trial. JAMA Dermatol. 2021;157:1165-73.
- [CrossRef] [Google Scholar]
- Abrocitinib versus Placebo or Dupilumab for atopic dermatitis. New Engl J Med. 2021;384:1101-12.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND) J Am Acad Dermatol. 2022;87:351-8.
- [CrossRef] [Google Scholar]
- Abrocitinib efficacy and safety in patients with moderate-to-severe atopic dermatitis: Results from phase 3 studies, including the long-term extension JADE EXTEND study. J Eur Acad Dermatol Venereol. 2023;37:2056-66.
- [CrossRef] [PubMed] [Google Scholar]
- Effective treatment of refractory alopecia areata in pediatric patients with oral abrocitinib. J Cosmet Dermatol. 2024;23:348-9.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of alopecia universalis with abrocitinib: A case report. J Dermatol Treatment. 2023;34:2242706.
- [CrossRef] [PubMed] [Google Scholar]
- A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. 2022;22:99-100.
- [CrossRef] [PubMed] [Google Scholar]
- Abrocitinib as a novel treatment for lichen sclerosus. Br J Dermatol. 2023;189:136-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-8.
- [CrossRef] [Google Scholar]
- Abrocitinib as a novel treatment for multiple skin disorders: 3 Case reports and a scoping review. Clin Cosmet Investig Dermatol. 2024;17:35-40.
- [CrossRef] [Google Scholar]
- Treatment of primary cutaneous lichenoid amyloidosis with abrocitinib: A pilot study in two cases. Int J Dermatol. 2023;62:e480-3.
- [CrossRef] [Google Scholar]
- A report of eruptive pruritic papular porokeratosis treated with abrocitinib. Clin Cosmet Investig Dermatol. 2023;16:2223-7.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of porokeratosis ptychotropica with abrocitinib. Int J Dermatol. 2024;63:1792-3.
- [CrossRef] [PubMed] [Google Scholar]
- Refractory Hailey-Hailey disease cleared with upadacitinib. JAAD Case Rep. 2023;41:64-7.
- [CrossRef] [PubMed] [Google Scholar]
- Off-Label uses of abrocitinib: Review of emerging therapeutic applications beyond atopic dermatitis. Life (Basel, Switzerland). 2024;14:1127.
- [CrossRef] [Google Scholar]
- Effect of abrocitinib in a patient with extensive necrobiosis lipoidica. J Eur Acad Dermatol Venereol. 2023;37:e1208-10.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid resolution of nonsegmental vitiligo in a patient treated with abrocitinib: A case report. SAGE Open Med Case Rep. 2024;12:2050313X241231527.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective observational study of oral abrocitinib and narrow-band ultraviolet-B in refractory progressive vitiligo. J Am Acad Dermatol. 2024;91:590-2.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-10.e2.
- [CrossRef] [PubMed] [Google Scholar]
- The Janus kinase 1 inhibitor abrocitinib for the treatment of oral lichen planus. J Eur Acad Dermatol Venereol 2023
- [CrossRef] [Google Scholar]
- Janus kinase inhibitors: A promising therapeutic option for allergic contact dermatitis. Cutis. 2023;111:92-105.
- [CrossRef] [PubMed] [Google Scholar]
- Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: A case report. Contact Dermatitis. 2022;87:542-4.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of atopic dermatitis with a predominant nipple involvement by abrocitinib during COVID-19 pandemic: A case report. J Asthma Allergy. 2023;16:789-92.
- [CrossRef] [Google Scholar]
- Tofacitinib for prurigo nodularis: A case report. Clin Cosmet Investig Dermatol. 2022;15:503-6.
- [CrossRef] [PubMed] [Google Scholar]
- Failure of dupilimab with severe prurigo nodularis that responded well to abrocitinib. Dermatitis. 2023;34:567.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of refractory prurigo nodularis with abrocitinib. Clin Case Rep. 2024;12:e8606.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: A nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-24.
- [CrossRef] [PubMed] [Google Scholar]
- Real-world experience of abrocitinib on difficult-to-treat hand eczema in Chinese patients. Acta Derm Venereol. 2024;104:adv39822.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of atopic hand and foot eczema with oral Janus kinase 1 inhibition. Dermatitis. 2023;34:560.
- [CrossRef] [PubMed] [Google Scholar]
- Real-world experience of abrocitinib treatment in patients with atopic dermatitis and hand eczema: Up to 28-week results from the BioDay registry. Acta Dermato Venereol. 2024;104:adv19454.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Netherton syndrome with abrocitinib. JAMA Dermatol. 2023;159:791-3.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of oral abrocitinib monotherapy in pityriasis rubra pilaris. J Eur Acad Dermatol Venereol. 2024;38:e920-2.
- [CrossRef] [Google Scholar]
- Treatment of rosacea with upadacitinib and abrocitinib: Case report and review of evidence for Janus kinase inhibition in rosacea. Front Immunol. 2024;15:1416004.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of granulomatous rosacea by JAK inhibitor abrocitinib: A case report. Clin Cosmet Investig Dermatol. 2023;16:3369-74.
- [CrossRef] [PubMed] [Google Scholar]
- JAK1 inhibitor abrocitinib for the treatment of steroid-induced rosacea: Case series. Front Med (Lausanne). 2023;10:1239869.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of abrocitinib in a patient with chronic actinic dermatitis. Am J Ther. 2024;31:e463-4.
- [CrossRef] [PubMed] [Google Scholar]
- Abrocitinib-A promising option for patients with refractory bullous pemphigoid. J Eur Acad Dermatol Venereol. 2024;38:e119-21.
- [CrossRef] [Google Scholar]
- Janus kinase inhibitor Abrocitinib as an Off-Label treatment for tattoo granuloma with uveitis (TAGU) Australas J Dermatol. 2024;65:297-9.
- [CrossRef] [PubMed] [Google Scholar]
- Oral abrocitinib in the treatment of granuloma annulare: A case report. J Dermatolog Treat. 2024;35:2313090.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of recalcitrant generalized granuloma annulare with the JAK inhibitor abrocitinib. J Dtsch Dermatol Ges. 2024;22:841-3.
- [CrossRef] [Google Scholar]
- Case report: Treatment of psoriasiform dermatitis in patients with malignancy. Front Med (Lausanne). 2024;11:1363405.
- [CrossRef] [Google Scholar]
- Post-hyaluronic acid filler reaction treated with abrocitinib: A case report. J Drugs Dermatol. 2024;23:1355-6.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of eosinophilic pustular folliculitis successfully treated with abrocitinib. J Dermatol. 2024;51:1694-7.
- [CrossRef] [Google Scholar]
- Oral abrocitinib in the treatment of refractory dissecting cellulitis of the scalp: A case report. J Dermatol. 2024;51:e329-30.
- [CrossRef] [Google Scholar]
- Real-time experience of abrocitinib for the treatment of mucous membrane pemphigoid: A case report. Patient Prefer Adherence. 2024;18:503-6.
- [CrossRef] [PubMed] [Google Scholar]
- A case of perioral dermatitis successfully treated with abrocitinib. Clin Cosmet Investig Dermatol. 2023;16:3035-8.
- [CrossRef] [PubMed] [Google Scholar]
- A case of persistent Pityriasis Rosea successfully treated by a short course of therapy with abrocitinib. Clin Cosmet Investig Dermatol. 2024;17:843-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cibinqo (Abrocitinib) official website. Pfizer; (n.d.) Available from: https://www.cibinqo.com [Last accessed on 2024 Dec 04]
- [Google Scholar]






