Translate this page into:
A review of comorbidities in atopic dermatitis
*Corresponding author: Andac Salman, Department of Dermatology, Acıbadem Mehmet Ali Aydınlar University School of Medicine Acıbadem Healthcare Group, Acıbadem Altunizade Hospital, Istanbul, Turkey. asalmanitf@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Apti Sengkioun O, Salman A. A review of comorbidities in atopic dermatitis. Indian J Skin Allergy. 2024;3:87-92. doi: 10.25259/IJSA_37_2024
Abstract
Atopic dermatitis (AD) is a chronic, recurrent inflammatory skin disorder that can affect people of all ages. Considering its high disease burden, it is traditionally linked with psychosocial comorbidities, including depression, anxiety, social anxiety, and substantially impaired quality of life. With advancements in understanding the pathogenesis of AD and the critical importance of comorbidities in managing patients with skin conditions, an increased number of studies have recently shown that a variety of comorbidities are more frequently seen in patients with AD. The recognition of these comorbidities is important for the optimum management of patients and the selection of systemic treatments. In this review, comorbidities in pediatric and adult patients with AD are summarized.
Keywords
Atopic dermatitis
Comorbidities
Psychiatric
Atopic
Autoimmune
INTRODUCTION
Atopic dermatitis (AD) is a prevalent skin disorder that affects approximately 20% of all young toddlers and 3–5% of adults.[1] AD has a complicated pathogenesis that includes both genetic and environmental risk variables. A major factor is the dysregulation of innate and adaptive immunity.[2] Furthermore, primary skin-barrier damage, such as frequent filaggrin gene mutations, epidermal inflammation,[3] and abnormal immune activation, is associated with an elevated risk of AD.[4] AD also has a significant systemic inflammatory component, which is directly related to the total inflammatory load in the skin. It may also contribute to AD comorbidities, such as cardiovascular disease progression.[5]
AD has very diverse clinical manifestations caused by genetic and environmental interactions that are still being elucidated.[6] It is linked with a significant burden on adults, children, their families and caregivers, and the healthcare system due to its chronicity.[7,8] Approximately one in two patients with AD described having itchy, dry, or scaling skin and irritated skin as the most bothersome symptoms. Approximately 10% of respondents rated skin soreness and sleep disturbance as the second most unpleasant symptoms.[9] AD is characterized by both atopic and non-atopic comorbidities such as ocular, psychiatric, cardiovascular, and autoimmune diseases and malignancies [Figure 1]. Patients with active diseases are linked to a higher risk of comorbidity development than those in remission, and the risk of comorbidity development increases with the severity of AD.[10] Since distinct comorbidities may be linked to various disease phenotypes, comorbidities are crucial because they raise the burden of AD. Regarding adverse effects, it could also influence the choice of therapy [e.g., cyclosporine is preferred in cases of conjunctivitis, dupilumab in asthma cases, methotrexate or Janus kinase inhibitors (JAKi) in cases of rheumatoid arthritis (RA), and JAKi in cases of alopecia areata (AA)].[11] As novel treatment modalities emerge,[12] the impact of comorbidities on the treatment selection will become increasingly relevant.
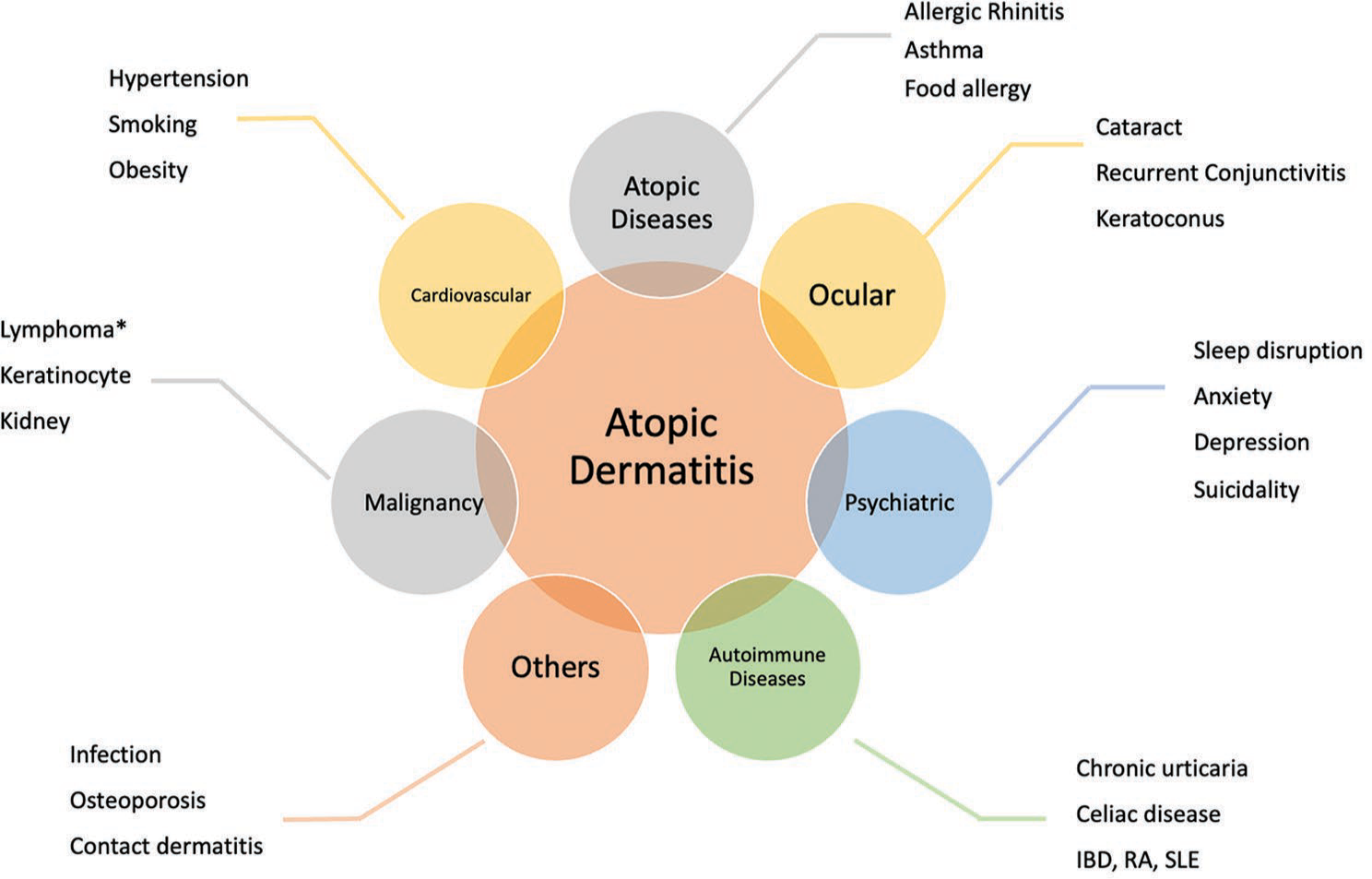
- Comorbidities of atopic dermatitis (IBD: Inflammatory bowel disease, RA: Rheumatoid arthritis, SLE: Systemic lupus erythematosus, *: Conflicting data).
METHODS
An extensive literature search in the PubMed/MEDLINE electronic database using the keywords “atopic dermatitis” OR “atopic eczema” AND “comorbidity” OR “comorbidities” OR “atopic” OR “sleep” “psychiatric” OR “neurological” OR “cardiovascular” OR “autoimmune” OR “ocular” OR “malignancy” OR “osteoporosis” OR “infections” OR “contact allergy” was conducted. The literature search was conducted in June 2024, and all types of English articles were included. To interpret comorbid conditions correctly, it is important to know augmented reality (AR) values as well as the relative risk (RR). RR estimations demonstrate the ratio of a specific outcome in the exposed group (AD patients) versus the unexposed group (controls). AR is more effective in presenting the true impact of an intervention, as RRs are not sensitive enough to distinguish between major and minor absolute changes.[11] Since systematic reviews (SRs) frequently do not include AR estimates, obtaining such information typically requires going into specific studies. This article describes current attempts to use SRs to determine the optimal search phrase algorithms for AD research.
COMORBIDITIES IN AD
Atopic comorbidities
AD has been considered the earliest clinical presentation of the “allergic march,” which also includes asthma, allergic rhinitis, and food hypersensitivity. Specific immunoglobulin E (s-IgE) levels and skin prick tests are used to define the allergic and non-allergic types of asthma and rhinitis that are linked to AD.[13,14] There is a well-established correlation between atopic diseases, which can be attributed to skin barrier failure caused by Th2/Th22 skewing.[15] Skin barrier dysfunction in AD patients lets allergens penetrate transcutaneously.[16] Rhinitis and asthma, at 27.5% and 40.5%, respectively, are the most common atopic comorbidities in adults with AD. These rates are higher in severe disease and childhood AD. In addition, filaggrin mutations were linked to increased asthma risk in the general population as well as an even greater risk in children who had AD and were sensitized to dust mites, grass, and cat dander.[17]
Food sensitivity and allergy rates are 28.6% and 24.1%, respectively, in people with AD.[11] Inquiring about gastrointestinal and respiratory atopic symptoms from patients is crucial as it influences the choice of medication. In addition, the prevalence of eosinophilic esophagitis is increased in patients with AD, which is associated with peripheral eosinophilia and higher IgE levels. Eosinophilic esophagitis is also closely associated with personal and family atopic predisposition (such as rhinitis and food allergies).[18]
Ocular comorbidities
Ocular disorders have been related to AD due to many variables, including allergic comorbidity, disease activity, infection, and dupilumab therapy.[19] The most common ocular findings in AD are anterior subcapsular cataracts, recurrent conjunctivitis, and keratoconus. AD patients may also experience keratitis, glaucoma, and posterior subcapsular cataracts, although these conditions are less prevalent.[20] In a recent SR, the overall prevalence of conjunctivitis in AD patients was found to be 31.7%, and no significant difference was observed between genders. In addition, the prevalence is higher in Europe. Conjunctivitis is more frequent in AD with active illness. The most prevalent subtypes of conjunctivitis among AD patients were rhinoconjunctivitis and allergic conjunctivitis (32.9% and 31.8%, respectively).[19]
The interaction of inflammation, mechanical stress, barrier disruption, and micro-organisms may predispose to ocular comorbidities.[21] Conjunctivitis, blepharitis, and keratitis are not just disease-related symptoms; the pooled proportion of side effects associated with the IL-4RA biologic dupilumab were 29.8%, 22.0%, and 1.4%, respectively.[19] In AD, ocular comorbidities may be interrelated and increases the disease burden. Detailed questioning and examination of the presence of ocular symptoms are crucial.
Psychiatric and neurological comorbidities
High prevalence of sleep disruption, social isolation, stigmatization, and severe pruritus have all been implicated with an increased risk of anxiety, depression, and suicidality in AD patients.[16] Psychiatric comorbidities (such as depression and anxiety) increase the disease burden in both adults and children. Nearly one in six AD patients experienced clinical depression, one in four experienced depressive symptoms, and one in eight experienced suicidal thoughts, according to an SR and meta-analysis.[22] In another meta-analysis where AD and psychiatric comorbidities were evaluated, the risk of depression and anxiety increased twofold, and the risk of suicidal thoughts was increased fourfold in AD.[23] Increased depression in AD may contribute to increased suicidality. On the other hand, many suicidal people do not fulfill the diagnostic criteria for depression.[24] Further, compared to children (4 of 6 studies (pooled odds ratio [OR], 1.31; 95% confidence interval [CI], 0.99–1.75), AD was linked to greater risks of depression in adults (16 of 20 studies [pooled OR, 2.08; 95% CI, 1.70–2.55]). Many studies show that depressive symptoms decrease with different topical, oral, systemic, and biological AD treatments. [25-27]
Adults and children with AD use antidepressants at a higher rate than those without; however, they were noticeably higher in AD adults. The difficulty of treating AD increases familial stress. While parental depression is more prevalent in children with AD than without (29.3% vs. 20.3%), it is not associated with AD in their offspring. Interestingly, according to a prospective single-center study, mothers had a higher risk of depression (OR 2.0 [95% CI, 1.1–3.6]) than fathers.[22] AD also negatively affects sleep quality. Sleep disorders can be seen in 47–80% of children and 33–90% of adults with AD. Moreover, as disease severity increases in AD, the prevalence and severity of sleep disorders is also increased. The “itching-scratching cycle,” which increases at night, is the main factor that disrupts sleep quality.[28] Especially in children, sleep disturbances can lead to neurodevelopmental delays and attention deficit hyperactivity disorder.[29] In a cohort study in which 145 children with AD were evaluated, a significant increase in the frequency of neurological diseases such as migraine and epilepsy was detected.[10]
Autoimmune comorbidities
There have been suggestions that AD is actually an “umbrella” term with an autoimmune profile at its core. Autoimmune diseases, such as dermatologic, gastrointestinal, and rheumatologic, are more prevalent in adult patients with AD, particularly smokers.[30] Patients with AD have 1.5–2 times higher rates of chronic urticaria, celiac disease, inflammatory bowel disease (IBD), systemic lupus erythematosus, and RA as compared to controls. [30-33]
Patients with vitiligo, particularly those with early onset or AA, seem to be more susceptible to AD. The OR of AA in patients with AD is raised tenfold.[11] In addition, patients with alopecia totalis and alopecia universalis were more susceptible than patients with patchy alopecia.[34] Although it is uncertain, the correlations may be explained by shared pathways, such as thymic stromal lymphopoietin[35] and Th17.[36] There may be common genetics behind the association; for example, filaggrin gene mutations in patients with AA were discovered to be related to more severe AA.[37] A large study showed an increased RR of RA, Crohn’s disease, and ulcerative colitis in patients with AD (RR: 1.72, 1.34 and 1.25, respectively).[38]
Cardiovascular comorbidities and association with metabolic syndrome
Although there are contradictory data about whether AD is connected with being overweight or obese, a meta-analysis revealed that patients with AD had a higher prevalence of being overweight and obese, and weight loss improved their eczema symptoms.[39] According to SRs, both active and passive smoking is connected with an increase in AD prevalence in children and adults. However, the impact of smoking on the severity of AD was not studied, and AD was not linked to maternal smoking during pregnancy.[40]
A recent SR that included 51 reviews found that approximately 1 in 6 patients with AD had hypertension, and the prevalence was higher in studies conducted in the USA. Moderate-to-severe AD, but not a mild disease, was associated with a higher odds of hypertension.[41] In AD, systemic inflammation, chronic sleep disturbance, higher rates of obesity, and the use of systemic corticosteroids and cyclosporine A increase the risk of hypertension. In a current study evaluating vascular inflammation and skin and blood immune biomarkers in AD through fludeoxyglucose F18 positron emission tomography-computed tomography, increased vascular inflammation and increased Th2 response in AD have been found to be associated with cardiovascular comorbidities.[42] There are conflicting data regarding the association of AD and metabolic syndrome. While a cross-sectional study showed an increased prevalence of metabolic syndrome components in AD patients, there are SRs in which AD was found to be associated only with central obesity.[43,44]
Malignancies
There are controversial studies on the risk of lymphoma in patients with AD. In an SR confirming a marginally elevated risk of lymphoma in severe AD, the importance of differential diagnosis with cutaneous T-cell lymphoma (CTCL) in adult-onset and severe AD is emphasized.[45] A multicenter study from Europe has reported a fourfold increase in the incidence rate (IR) of lymphoma in children treated with tacrolimus and a less than twofold increase in the IR of CTCL in adults who received topical calcineurin inhibitor (TCI) treatment compared with users of topical corticosteroids.[46] In addition, a 10-year follow-up cohort study in AD children using topical pimecrolimus for a minimum of 6 weeks reported a 2.6-fold increase in the standardized incidence ratio for lymphoma.[47] However, moderate-certainty evidence from current studies suggests that TCIs do not increase the risk of cancer in patients with AD and that TCIs are safe to use in their optimal treatment.[48] In a recent retrospective observational case series of adult AD patients treated with dupilumab, a reversible and benign lymphoid reaction mimicking CTCL was observed.[49] In an SR and meta-analysis based on population-based cohort studies, it has been suggested that AD increases the risk of keratinocyte and renal cancer but plays a preventive role in lung and respiratory system cancers.[50]
Others (infection, osteoporosis, and contact dermatitis)
Disturbances in the skin barrier, defects in cutaneous innate immunity due to increased type 2 inflammation, lower levels of antimicrobial peptides, colonization with Staphylococcus aureus, and changes in cutaneous microbiome all contribute to an increased rate of infections in AD.[51] Impaired cellular immunity leads to herpes simplex, varicella zoster, verrucae, and molluscum contagiosum infections in AD patients.[52] Moreover, the increased frequency of bacterial infections is also known; in an SR and meta-analysis, a 20-fold increased S. aureus colonization has been reported.[53]
AD was linked to increased odds of infections involving the ear, throat, and urinary tract, and extracutaneous infections in an SR that included seven studies.[54] Two studies found a correlation between AD and increased risks of meningitis and infected endocarditis.[54]
AD and hand eczema are both chronic and recurrent inflammatory pruritic skin diseases. Experimental investigations have demonstrated that AD skin loses more transepidermal water and absorbs more irritants and allergens than healthy skin.[55] In a meta-analysis including 26 studies, the prevalence of hand eczema was found to be three- and four-fold higher in AD patients compared to controls.[56] Furthermore, an SR on the correlation with allergic contact dermatitis (ACD) revealed a 1.5-fold increase in the risk of ACD in patients with milder types of AD. Contact sensitivity to compositae mix and chromium was more common in AD patients, but there was no significant difference between AD and non-AD people in terms of the overall prevalence of contact sensitization to common allergens.[57] Even if AD is associated with a higher risk of ACD than the general population, the magnitude of this association is rather minor. That is, only a small percentage of AD patients appear to develop ACD.[16] Therefore, patch testing is not recommended for all AD patients. According to expert consensus opinions, patch testing should be considered in AD patients “with dermatitis that does not improve with topical therapy, atypical/changing distribution of eczema, or a pattern suggestive of ACD, therapy-resistant hand eczema in the working population, late-onset AD, and/or before starting systemic immunosuppressants for dermatitis treatment.”[58]
Systemic inflammation, smoking, physical inactivity, Vitamin D deficiency, and corticosteroid or psycholeptic use have been reported to increase the risk of osteoporosis.[5,40,59]
According to recent AD guidelines from the American Academy of Dermatology, adult AD is linked to both bone fractures (moderate-quality evidence) and osteoporosis (high-quality evidence).[60]
Biomarkers of comorbidities
Over the past few decades, numerous potential AD biomarkers have been put out. Thymus and activation-regulated chemokine, for example, has been found to be a biomarker of the severity of AD. It was proposed that C-C motif chemokine 22 (CCL22) might serve as a biomarker of therapy response.[61,62] However, there is a shortage of high-quality studies examining biomarkers linked to the development of comorbidities, and no biomarkers are approved for clinical use in monitoring patients of AD. In a recent SR, where 56 articles were included, and 146 candidate biomarkers were evaluated, the most frequently reported biomarkers were filaggrin mutations and allergen-s-IgE. Some of the findings are intriguing, particularly in the case of asthma, where s-IgE and/or skin prick tests may help identify whether patients are at risk of acquiring asthma or wheezing.[63] However, the methodology and results are highly heterogeneous, and the risk of bias is medium/high. Further independent validation studies should use standardized evaluation procedures to account for confounding variables.
Importance of comorbidities in AD management
One factor that determines disease follow-up in AD is the impact of comorbidities on the burden of disease. Comprehensive examinations may also detect comorbidities accompanying AD and be predictive of AD treatment selection (e.g., cyclosporin may improve conjunctivitis, and sarilumab may improve asthma).
In the case of comorbid autoimmune diseases such as IBD, RA, AA, or severe asthma, specific preference for JAKi or biologics provides significant benefit in AD patients with comorbidities.[11] Nevertheless, the use of JAKi generally has been linked to an increased risk of cancer and cardiovascular disease.[64] However, in a study conducted with JAKi (abrocitinib, baricitinib, and upadacitinib) at different doses, no increase in the risk of serious infection, non-melanoma skin cancer, other cancers, major adverse cardiovascular events, venous thromboembolism, and nasopharyngitis were detected.[65] Although there are conflicting data on this subject, this should be taken into consideration when choosing treatment for this group of patients.
CONCLUSION
Numerous atopic comorbidities, such as food sensitivity and allergies, asthma, allergic rhinitis, as well as nonatopic comorbidities, depression, suicidality, cutaneous and extracutaneous infections, and cardiovascular disease, have been linked to AD. There is a rising interest in AD comorbidities, which can have a wide range of implications for patient care. Novel treatments to be developed in AD ideally should be specific for not only the treatment of the disease but also for the accompanying comorbid conditions. Future studies are needed to elucidate the exact mechanisms of the relationships between AD and comorbidities and to guide optimal therapies. This review outlines the most recent advancements in the comorbidities and burden of AD.
Acknowledgment
For his unwavering support and encouragement special thanks to Ege Sengun.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284-93.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis: The skin barrier and beyond. Br J Dermatol. 2019;180:464-74.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of unique proteomic signatures in allergic and non-allergic skin disease. Clin Exp Allergy. 2017;47:1456-67.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792-9.
- [CrossRef] [PubMed] [Google Scholar]
- Moderate-to-severe atopic dermatitis patients show increases in serum C-reactive protein levels, correlating with skin disease activity. F1000Res. 2017;6:1712.
- [CrossRef] [PubMed] [Google Scholar]
- Unraveling the complexity of atopic dermatitis: The CK-CARE approach toward precision medicine. Allergy. 2020;75:2936-8.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic eczema: Burden of disease and individual suffering-results from a large EU study in adults. J Eur Acad Dermatol Venereol. 2019;33:1331-40.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of atopic dermatitis in paediatric patients: An international cross-sectional study. Br J Dermatol. 2024;190:846-57.
- [CrossRef] [PubMed] [Google Scholar]
- Patient burden and quality of life in atopic dermatitis in US adults. Ann Allergy Asthma Immunol. 2018;121:340-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidities in childhood atopic dermatitis: A population-based study. J Eur Acad Dermatol Venereol. 2024;38:354-64.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidities of atopic dermatitis-what does the evidence say? J Allergy Clin Immunol. 2023;151:1155-62.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging systemic treatment options in atopic dermatitis. Balkan Med J. 2024;41:239-47.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinitis prevalence and association with atopic dermatitis. Ann Allergy Asthma Immunol. 2021;127:49-56.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of asthma in patients with atopic dermatitis: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;84:471-8.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes: IL-22 affects filaggrin expression at multiple levels. Br J Dermatol. 2011;165:492-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-51.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of disease associated with filaggrin mutations: A population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-7.e9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of atopic comorbidities in eosinophilic esophagitis: A case-control study of 449 patients. J Am Acad Dermatol. 2017;76:559-60.
- [CrossRef] [PubMed] [Google Scholar]
- Bidirectional association between atopic dermatitis, conjunctivitis, and other ocular surface diseases: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;85:453-61.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60:44-7.
- [CrossRef] [Google Scholar]
- Ocular involvement in atopic disease: A review. Curr Opin Ophthalmol. 2018;29:576-81.
- [CrossRef] [PubMed] [Google Scholar]
- Association between atopic dermatitis, depression, and suicidal ideation: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:402-10.
- [CrossRef] [PubMed] [Google Scholar]
- Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:448-56.e30.
- [CrossRef] [PubMed] [Google Scholar]
- Suicidal ideation and suicide attempts in general medical illnesses. Arch Intern Med. 2000;160:1522.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of a 5-HT1a receptor agonist in atopic dermatitis: Anxiolytic drugs for atopic dermatitis. Clin Exp Dermatol. 2010;35:835-40.
- [CrossRef] [PubMed] [Google Scholar]
- Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74:491-8.
- [CrossRef] [PubMed] [Google Scholar]
- Seasonality and BDNF polymorphism influences depression outcome in patients with atopic dermatitis and psoriasis. World J Biol Psychiatry. 2017;18:604-14.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep disturbances and atopic dermatitis: Relationships, methods for assessment, and therapies. J Allergy Clin Immunol Pract. 2021;9:1488-500.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213-9.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-80.e1.
- [CrossRef] [PubMed] [Google Scholar]
- The bidirectional association between inflammatory bowel disease and atopic dermatitis: A systematic review and meta-analysis. Dermatology. 2020;236:546-53.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis is a risk factor for rheumatoid arthritis: A systematic review and meta-analysis. Dermatitis. 2021;32:S15-23.
- [CrossRef] [PubMed] [Google Scholar]
- Association of atopic dermatitis with an increased risk of systemic lupus erythematosus: A systematic review and meta-analysis. J Postgrad Med. 2021;67:139-45.
- [CrossRef] [PubMed] [Google Scholar]
- Association of vitiligo and alopecia areata with atopic dermatitis: A systematic review and meta-analysis. JAMA Dermatol. 2015;151:522.
- [CrossRef] [PubMed] [Google Scholar]
- The role of thymic stromal lymphopoietin in the immunopathogenesis of atopic dermatitis. Clin Exp Allergy. 2011;41:1515-20.
- [CrossRef] [PubMed] [Google Scholar]
- Immunology of atopic eczema: Overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-82.
- [CrossRef] [PubMed] [Google Scholar]
- Loss-of-function mutations in the Filaggrin gene and alopecia areata: Strong risk factor for a severe course of disease in patients comorbid for atopic disease. J Invest Dermatol. 2007;127:2539-43.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137:130-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association of atopic dermatitis with being overweight and obese: A systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606-16.e4.
- [CrossRef] [PubMed] [Google Scholar]
- Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:1119-25.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Association between atopic dermatitis and hypertension: A systematic review and meta-analysis. Br J Dermatol. 2022;186:227-35.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular inflammation in moderate-to-severe atopic dermatitis is associated with enhanced Th2 response. Allergy. 2021;76:3107-21.
- [CrossRef] [PubMed] [Google Scholar]
- Association between atopic dermatitis and the metabolic syndrome: A systematic review. Dermatology. 2018;234:79-85.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis and the metabolic syndrome: A cross-sectional study of 116 816 patients. J Eur Acad Dermatol Venereol. 2019;33:1762-7.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: A systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- [CrossRef] [PubMed] [Google Scholar]
- A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation-JOELLE study) Clin Epidemiol. 2018;10:299-310.
- [CrossRef] [PubMed] [Google Scholar]
- Association between malignancy and topical use of pimecrolimus. JAMA Dermatol. 2015;151:594.
- [CrossRef] [Google Scholar]
- Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: A systematic review and meta-analysis. Lancet Child Adolesc Health. 2023;7:13-25.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab-associated lymphoid reactions in patients with atopic dermatitis. JAMA Dermatol. 2023;159(11):1240-7.
- [CrossRef] [PubMed] [Google Scholar]
- Noncutaneous and cutaneous cancer risk in patients with atopic dermatitis: A systematic review and meta-analysis. JAMA Dermatol. 2020;156:158.
- [CrossRef] [PubMed] [Google Scholar]
- The infectious complications of atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126:3-12.
- [CrossRef] [PubMed] [Google Scholar]
- Multimorbidity and mortality risk in hospitalized adults with chronic inflammatory skin disease in the United States. Arch Dermatol Res. 2020;312:507-12.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: A systematic review and meta-analysis. Br J Dermatol. 2016;175:687-95.
- [CrossRef] [PubMed] [Google Scholar]
- Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:904-12.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892-908.
- [CrossRef] [PubMed] [Google Scholar]
- The association between atopic dermatitis and hand eczema: A systematic review and meta-analysis. Br J Dermatol. 2018;178:879-88.
- [CrossRef] [PubMed] [Google Scholar]
- Association between atopic dermatitis and contact sensitization: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-8.
- [CrossRef] [PubMed] [Google Scholar]
- A pragmatic approach to patch testing atopic dermatitis patients: Clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-92.
- [CrossRef] [PubMed] [Google Scholar]
- The role of Vitamin D in atopic dermatitis. Dermatitis. 2015;26:155-61.
- [CrossRef] [PubMed] [Google Scholar]
- American Academy of Dermatology guidelines: Awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022;86:1335-6.e18.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr Opin Allergy Clin Immunol. 2015;15:453-60.
- [CrossRef] [PubMed] [Google Scholar]
- Improving evaluation of drugs in atopic dermatitis by combining clinical and molecular measures. J Allergy Clin Immunol Pract. 2020;8:3622-5.e19.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers associated with the development of comorbidities in patients with atopic dermatitis: A systematic review. Allergy. 2023;78:84-120.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancers under Janus kinase inhibitors: A World Health Organization drug safety database analysis. Therapie. 2022;77:649-56.
- [CrossRef] [PubMed] [Google Scholar]
- The safety of systemic Janus kinase inhibitors in atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2024;38:52-61.
- [CrossRef] [PubMed] [Google Scholar]







