Translate this page into:
A review of biological agents and small molecules in the management of atopic dermatitis
*Corresponding author: Aishwarya Ashokbhai Ramani, Department of Dermatology, Venereology and Leprosy, Gujarat Adani Institute of Medical Sciences, Bhuj-Kutch, Gujarat, India. draishwarya0204@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ramani AA, Bose S, Madke B, Prakashey AR, Ganjre S. A review of biological agents and small molecules in the management of atopic dermatitis. Indian J Skin Allergy 2023;2:51-9.
Abstract
Atopic dermatitis (AD) is a recurrent, chronic, and non-infectious inflammatory dermatoses characterized by persistent itching of the skin. It has multifactorial causes characterized by a tetrad of a (i) genetic predisposition, (ii) disturbed epidermal barrier, (iii) immune dysregulation, and (iv) deranged skin microbiome. At present, treatment is aimed at maintaining the epidermal barrier function by the use of emollients, sedative, and non-sedative antihistamines targeting the itch pathway, immunosuppressants in the form of steroids as well as steroid sparing agents to control the chronic persistent inflammatory response. However, newer emerging therapy in the form of biologics gives a promising approach to control the chronic, persistent inflammatory response by targeting the specific cytokines involved in pathogenesis of AD.
Keywords
Atopic dermatitis
Biologics
Biological agents
Dupilumab
Janus kinase Inhibitors
INTRODUCTION
Atopic dermatitis (AD), also known as atopic eczema, is a common inflammatory skin condition that can be chronic and recurrent and has a large social, psychological, and economic impact on patients all over the world, of which 2.1–4.9% are adults and 15–20% are children.[1,2] Conventional management of AD consists of topical therapy in the form of topical corticosteroids (TCS) and calcineurin inhibitors for mild AD. Topical steroid can be combined with phototherapy such as ultraviolet (UV) A and narrow band UV B.
Immunosuppressants such as short-term oral steroids with steroid sparing agents, of which cyclosporine A is most often used, are required for the treatment of moderate to severe AD.[3]
Targeted therapies for AD, such as interleukin (IL) IL-4/13 inhibitors, Janus kinase (JAK) inhibitors, and IL-13 inhibitors can be used topically as well as systemically. These are used for moderate-to-severe AD, refractory to the conventional forms of systemic treatment.
At present, crisaborole ointment, tralokinumab, and dupilumab have been approved by the US Food and Drug Administration (FDA), for use in patients with AD.[4,5]
In this review, we shall be discussing the various biologic agents used for the treatment of AD, and also their relevance/ need in comparison to the conventional therapies.
Apart from biologics, newer targeted therapies, that include JAK inhibitors and phosphodiesterase 4 inhibitors, are in different stages of trials for the management of AD.
LITERATURE SEARCH STRATEGY
The review aims to discuss the safety, efficacy, and mode of action of various biologic agents that are approved recently, and some of which are currently in Phases 2 and 3 clinical trials for treatment of moderate-to-severe AD.
A comprehensive search was done using PubMed, Google, EMBASE, Cochrane, and MEDLINE on studies published till date on biologic agents used in AD with emphasis on the dosage, formulations, safety, and adverse effects of each biologic agent. Only English language articles were considered. The keywords such as AD, biologics, JAK inhibitors, clinical trials, randomized controlled trials, studies, dupilumab, lebrikizumab, tralokinumab, mepolizumab, reslizumab, secukinumab, MOR106, baricitinib, and delgocitinib were used. Around 56 articles were selected after removing duplication and articles having no relevant information.
Clinical as well as randomized double-blinded or single-blinded controlled trials, open-label studies, retrospective studies, case series, reviews, and case reports on the use of biologics in AD, including their adverse effects, were screened and documented evidence was prepared, analyzed, and presented in a concise fashion to highlight the various important biologics used in AD. Studies conducted till April 2022 were considered for this review. Few clinical trials mentioned in this review are still awaiting approval.
PATHOGENESIS OF ATOPIC DERMATITIS
Normal skin barrier is made of corneocytes and tight junctions in the outermost layer of epidermis. The function of this barrier is to protect environmental microbes, allergens, and irritants and prevent transepidermal water loss.
1) In AD, multiple factors contribute to impaired skin barrier function, as shown in [Figure 1].
Lack of fatty acids, notably long-chain fatty acids and ceramides
Down regulation of tight junction and epidermal differentiation proteins (particularly filaggrin)
Ineffective antimicrobial peptide (AMP) responses to bacterial infections, such as Staphylococcus aureus, also weaken the barrier against microorganisms.[6]
2) The keratinocytes are disturbed by scratching, or environmental triggers leading to the following cascade[7]
Chemokines: CCL17/thymus and activation regulated chemokine and CCL22/macrophage-derived chemokine, Alarmins: such as thymic stromal lymphopoietin (TSLP), IL-1b, IL-25, and IL-33 from the keratinocytes.
Activation of skin-resident Type 2 innate lymphoid cells and T helper 2 (Th2) cells inflammatory response
Release the inflammatory cytokines of AD, IL-13, and IL-4.
3) Dendritic cells express OX40 ligand on stimulation by TSLP
Release of IL-4, I-5, IL-13, and I-31, by binding of OX40 on T cells.
In AD, IL-36 subfamily and its receptor are localized to the skin (and bronchial epithelium) are over expressed.
4) Exogenous (environmental) pruritogens triggering itch sensation (for example, pathogens, irritants, toxins, and allergens) and endogenous pruritogens derived from keratinocytes and immune cells (e.g., proteases, neuropeptides, lipids, and cytokines) cause activation of a variety of receptors on itch sensory neurons, which include G-protein coupled receptors, transient receptor potential channels, and cytokine receptors.
This leads to the generation of action potentials and local production of mediators of neurogenic inflammation, such as calcitonin gene related peptide and substance P.[8]
Activation of itch pathway and transmission of itch sensation through unmyelinated C-fibers of the dorsal root ganglia takes place.
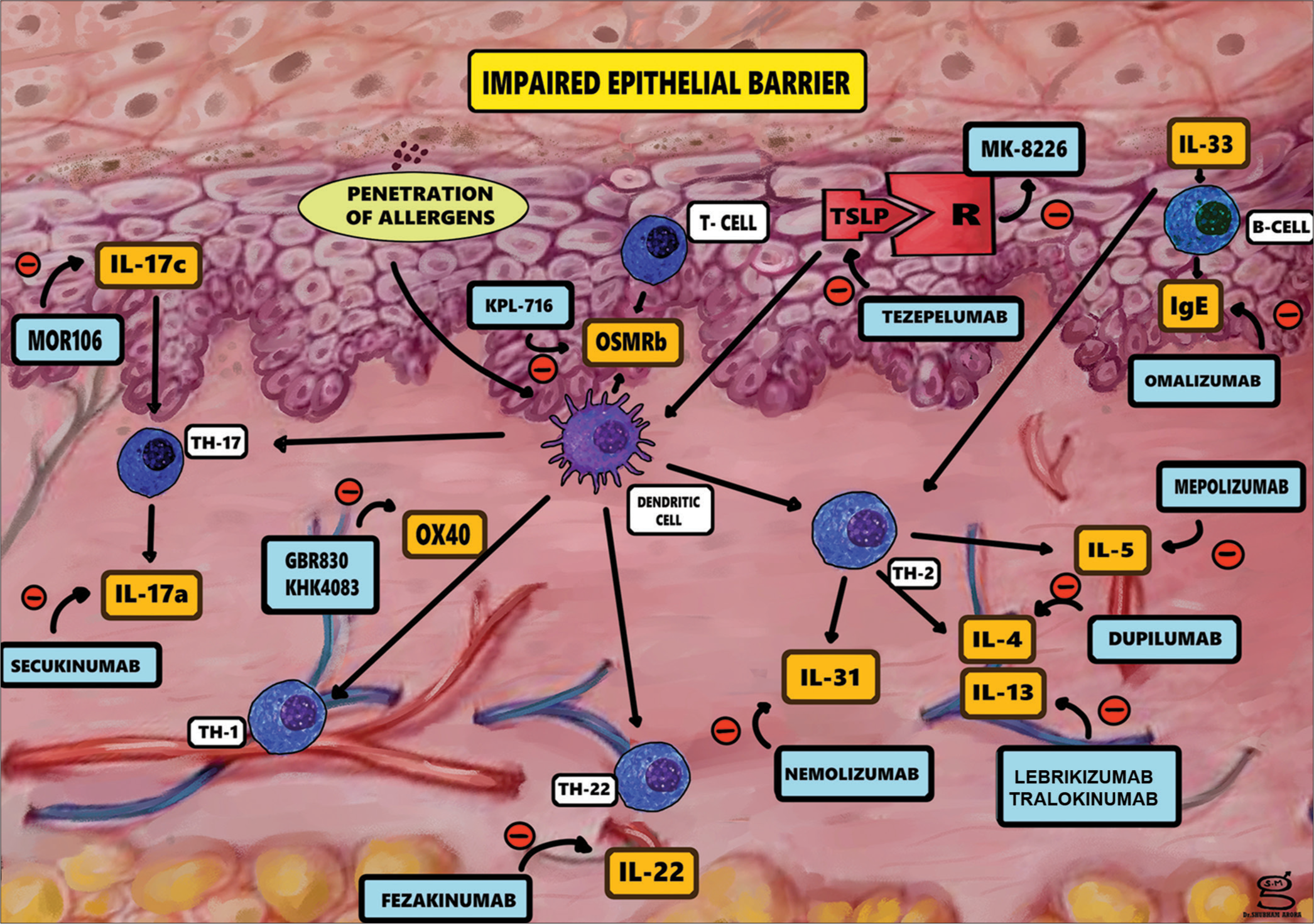
- Pathogenesis of atopic dermatitis and possible therapeutics agents.
Other than causing inflammation, Type 2 cytokines (IL-31, IL-33, and TSLP) also directly activate itch sensory neurons by binding to the neural receptors and then signaling through the Janus kinase- signal transducers and activators of transcription (JAK-STAT) pathway.
Although the activation of the IL-4 receptor alpha (IL-4Ra) on neurons is also JAK-STAT dependent, it is not responsible for directly causing itch, rather, it augments the response of neurons to Type 2 cytokine pruritogens.[9]
As such, targeting these alarmins, Th2 cytokines, or their receptors may both reduce inflammation and suppress the itch of AD.
Classification of biologic agents based on the mechanism of action
Biologic agents are sourced from living organisms and are engineered to target certain specific mediators of inflammation such as the cytokines.
Most biologics are still under clinical trials and awaiting approval for use in AD.
Dupilumab is the systemic biologic agent which was approved in 2017, for the use in adults with AD and in children aged 6 and above. Biologic agents have been classified according to their mode of action [Table 1].
| Biological agent | Targeted cytokine | Role of cytokine in AD | Produced from (source) |
|---|---|---|---|
| Dupilumab | IL-4 | (a) Th2 cell differentiation, Eosinophil and IgE production. Promotes inflammation of epidermis, acanthosis, and fibrosis (b) Reduces AMP |
Th2 cells |
| Lebrikizumab, tralokinumab | IL-4/13 | Promotes inflammation of epidermis | Th2 cells |
| Mepolizumab, reslizumab | IL-5 | Causes eosinophilia | Th2 cells |
| Secukinumab, MOR106 | IL-17 | (a) Promotes Th2 cells to produce IL-4 (b) Differentiates B cells to IgE producing plasma cells (c) Increases production of IL-8, TSLP, CXCL10, and AMP |
Th1, Th2, Th17 and ILC |
| Fezakinumab | IL-22 | Promotes inflammation of epidermis | Th22 cells |
| Nemolizumab | IL-31 | Increases pruritus and inflammation | Th2 cells, mast cells |
| Tezepelumab | TSLP (Thymic Stromal Lymphopoietin) | Keratinocytes | Promotes T cell differentiation by acting on dendritic cell, Promotes Th2 Response |
| GBR 830 KHK4083 | OX40 | Induction and maintenance of Th2 response | Th2, CD8+cells |
IL: Interleukin, Th2: T helper 2, IgE: Immunoglobulin E, AD: Atopic dermatitis, AMP: Antimicrobial peptide, TSLP: Thymic stromal lymphopoietin, ILC: Innate lymphoid cells, CXCL10: Chemokine interferon-γ inducible protein 10 kDa
In addition, biological agents targeting immunoglobulin E (IgE) antibody (omalizumab, ligelizumab, quilizumab) are being used in management of AD.
Newer treatment modalities include JAK Inhibitors (tofacitinib, ruxolitinib, delgocitinib, baricitinib, and upadacitinib) that are being increasingly used in the treatment of AD. A concise summary of the JAK inhibitors used in AD is given in Table 2. The FDA has approved three JAK inhibitors for the use in atopic dermatitis- these include ruxolitinib, upadacitinib and abrocitinib.
| JAK inhibitor name | Target molecule | Mode of drug delivery | Dose | Frequency | Study phase |
|---|---|---|---|---|---|
| Abrocitinib[34-37] | JAK1 | Oral | 100 mg, 200 mg | OD | Approved |
| Baricitinib[38-41] | JAK1, JAK2 | Oral | 4 mg (2 mg) | OD | Approved |
| Upadacitinib[42-44] | JAK1 | Oral | 7.5 mg, 15 mg, 30 mg | OD | Approved |
| Delgocitinib[45-48] | JAK1, JAK2, JAK3, TYK2 | Topical (ointment) | 0.5% | BD | Approved |
| Ruxolitinib[49-52] | JAK1, JAK2 | Topical (cream) | 0.15%, 0.5%, 1.5% | BD | Approved |
| Brepocitinib[49] | JAK1/TYK2 | Topical(cream) | 1% | QID, BD | Phase 2a |
| Tofacitinib[53] | JAK1, JAK3 | Topical(ointment) | 2% | BD | Phase2a |
| Cerdulatinib[54] | JAK1 | Oral | 20 mg, 40 mg, 80 mg | OD | Phase1b |
| Tofacitinib[55] | JAK1 | Oral | 100 mg, 200 mg | OD | Phase3 |
| Gusatinib[56] | JAK1 | Oral | 20 mg, 40 mg, 80 mg | OD | Phase1b |
| SNA-125 | JAK3, TrkA | Topical | Planned |
JAK: Janus Kinase, TrKa: Tropomyosin kinase A receptor, TYK2: Tyrosine kinase 2 receptor, QID: Four times a day, BD: Twice a day, OD: Once a day, SNA: Sympathetic nerve activity
Anti-IL-4 agents
Dupilumab was first approved by the FDA in 2017 and the European Medicines Agency (EMA) in 2019 for use in moderate-to-severe AD in age group >12 years and has recently been approved for pediatric patients of 6 months age.[10-12]
Mechanism of action
It is fully humanized monoclonal antibody of class IgG 4 antagonizing IL-4 alpha receptor which is shared by IL-4 and 13 blocking the synergistic effects of IL-4 and IL-13 in atopic inflammation and further inhibits release of pro-inflammatory cytokines, chemokines and IgE.
Pharmacokinetics
After a single subcutaneous injection, it achieves a maximum drug concentration (T-max) in 3–7 days.
Steady state concentration of the drug is achieved at 16th week.
Bioavailability
The bioavailability of dupilumab is 61–64%. It is primarily distributed in the vascular.
Elimination
At a higher concentration elimination occurs through nonsaturable proteolytic pathway, while at lower concentrations saturable IL-4Ra target mediated elimination takes place.
Dosage and route of administration
Dupilumab is available as a 300 mg/2 mL solution in a single-dose prefilled syringe with or without a needle shield.
The recommended loading dose loading dose (LD) is 600 mg followed by maintenance dose 300 mg, either weekly or every alternate week for 16 weeks.
Dupilumab is given as a subcutaneous injection to the upper arm, thigh, or abdomen, avoiding two inches around the navel. Due care should be taken not to administer into skin that is tender, bruised or scarred. The site of injection must be rotated with each injection.
The prefilled syringe is allowed to reach the room temperature for 45 min before injecting.[13,14] If the patient misses the dose, it should be administered within 7 days and the original schedule is resumed; however, if the dose is not administered in 7 days, next dose of original schedule is to be awaited.
Drug interactions
Live vaccines should be avoided while receiving dupilumab. These vaccines should be administered atleast 4 weeks prior to initiating dupilumab, Monitoring of therapy is required with cytochrome P450 (CYP450) substrates such as warfarin, cyclosporine as increased levels of IL4, IL13 alter formation of CYP450 enzymes.
Use in pregnancy and lactation
At present, no significant data is available, but since IgG can cross-placental barrier and also be secreted in breast-milk, dupilumab may be transferred to fetus/breastfed infant.
Geriatric population
No significant data available.
Major adverse events
Table 3 gives a summary of the biologic drug along with the trials and results, including adverse effects, followed by ongoing trials.
| Biologic | Target IL | Trial phase | Age group | Dosage | Results | Adverse events | Level of evidence | Ongoing trials if any | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dupilumab | IL-4R alpha | SOLO continue phase 3 | >18 years |
LD: 600 mg S.C. MD: 300 mg weekly |
EASI-75 | Dupilumab Weekly or Q2W: 71.6% Q4W: 58.3% Q8W: 54.9% |
Placebo Placebo: 30.4% |
Conjunctivitis Injection site reactions |
A.1a | Phase 2: NCT03861455 NCT03346434 Phase 3: NCT02612454 NCT01949311 Phase 4: NCT03667014 NCT03389893 NCT03293030 NCT04447417 NCT04033367 |
| Phase 3 (AD1526) | 12–18 years | LD: 600 mg S.C. MD: 300 mg weekly, or 2 weekly or 4e weekly |
IGA | Dupilumab Q2W: 24.4% Q4W: 17.9% |
Placebo Placebo: 2.4% |
Conjunctivitis Injection site reactions |
||||
| EASI | Q2W: 41.5% Q4W: 38.1% |
Placebo: 8.2% | ||||||||
| Phase 3 | 6–11 years | LD: 200 mg MD: 100 mg 2 weekly OR LD: 400 mg MD: 200 mg 2 weekly |
- | - | - | Ongoing trial NCT03345914 |
||||
| Lebrikizumab | IL 4/13 | Phase 2B (NCT03443024) |
>18 years | 1) LD: 250 mg MD: 125 mgQ4W 2) LD: 500 mg, MD: 250 mg Q4W 3) LD: 500 mg MD: 250 Q2W Placebo Q2W |
EASI | Lebrikizumab 125 mg Q4W: 62.3% 250 mg Q4W: 69.2% 250 mg Q2W 72.1% |
Placebo Placebo: 41.1% |
Upper respiratory tract infection Nasopharyngitis Headache Injection site pain Herpes infection Conjunctivitis |
B,2b | Phase 3 NCT04146363 NCT04178967 NCT04392154 NCT04250350 NCT04250337 |
| Tralokinumab | IL-13 | Phase 2b | 18–75 | 45 mgQ2W 150 mg Q2W 300 mgQ2W Placebo Q2W through S.C. |
EASI score 150 mg: −4.36 300 mg: −4.94 |
Nasopharyngitis Upper respiratory tract infection Headaches |
B, 2b | |||
| Phase 3 (ECZTRA 1, 2) | 300 mg Q2W placebo Q2W If clinical response achieved at week 16: 300 mg Q2W 300 mg Q4W placebo Q2W SC route |
ECZTRA 1 | Tralokinumab | Placebo | Upper respiratory infections Conjunctivitis keratitis malignancies pruritus headache Eczema herpeticum |
|||||
| IGA EASI 75 |
Q2W: 51.3% Q4W: 38.9% Q2W: 59.6% Q4W: 49.1% |
Placebo: 47.4% Placebo: 33.3% |
||||||||
| ECZTRA 2 | Tralokinumab | Placebo | ||||||||
| IGA EASI 75 |
Q2W: 59.3% Q4W: 44.9% Q2W: 55.8% Q4W: 51.4 |
Placebo: 25% Placebo 21.4% |
||||||||
| Nemolizumab | IL31R alpha |
Phase 2b | >18 years | LD: 60 mg MD: 30 mg Q4W S.C |
At 24 wk EASI score % change |
Nemolizumab 68.8% |
Placebo Placebo: 52.1% |
Dose dependent Asthma exacerbations, Atopic dermatitis, Nasopharyngitis |
A,1b | Phase 2 NCT03921411 NCT04365387 Phase 3 NCT03985943 NCT03989349 NCT03989206 |
| Phase 3 | >13 years | 60 mg Q4W S.C. | At 8 weeks EASI: 24.2 (16.9–36.1) At 16 weeks EASI : −45.9±3.3−33.2±4.7 |
Nasopharyngitis Atopic dermatitis Injection site reactions |
- | |||||
| Tezepelumab | TSLP | Phase 2a | 12–75 years | Failed to reach statistical significance 280 mg: 64.7% versus placebo: 48.2% | Injection-site erythema) | Phase 2b NCT03809663 |
||||
| ISB 830 | OX40 | Phase 2a | >18 years | LD: 600 mg MD: 300 mg Q4W LD: 150 mg MD: 75 mg Q2W LD: 1200 mg MD: 600 mgQ2W |
At 16 wk EASI score % change |
ISB 830 63.0% |
Placebo Placebo: 63% |
Headache Atopic dermatitis Nasopharyngitis |
B,2b | Phase 2b NCT03568162 |
| Fezakinumab | IL22 | Phase2a | LD: 600 mg MD: 300 mg Q2W IV route |
At12 wk | Fezakinumab 65.5% |
Placebo Placebo: 12% |
Headache Nasopharyngitis |
- | - | |
Anti-IL-13 agents
Tralokinumab
Mechanism of action
Tralokinumab binds to the IL-13 on an epitope that overlaps with the binding site for IL-13Ra receptors. Thus, it blocks IL-13 from binding to both IL-13Ra1 and IL-13Ra2 receptors.
However, it must be noted that IL-13 has higher binding affinity than tralokinumab to the IL-13Ra2 receptor; so free IL-13 can still bind to the receptor.[15,16]
Dosage and route of administration
It is given at a dose of 600 mg subcutaneous followed by 300 mg every other week.
In patients weighing <100 kg treated for 16 weeks and in those who achieve clear or almost clear skin, one may consider a dose of 300 mg SC every 4 weeks.
Pharmacokinetics
After a single subcutaneous injection, it achieves maximum concentration within 5–8 days and steady state is reached by 16 weeks. The bioavailability of tralokinumab is 76% while its half-life is 3 weeks.
Clinical trial data
Tralokinumab was studied in Phase III clinical trials and reached its primary endpoints at week 16 in trials (ECZTRA 1 and 2 as monotherapy and ECZTRA 3 with concomitant TCS, with response which was maintained over time. Significant improvements in secondary outcomes, such as pruritus and dermatology quality of life, were demonstrated in trials.[16,17]
Lebrikizumab
Mechanism of action
Lebrikizumab acts by binding to the IL-13 on an epitope that overlaps with the binding site of the IL-4Ra receptor, blocking the heterodimerization of the IL-4Ra/IL-13Ra1 subunits, though IL-13 can still bind to IL-13Ra2.
Dosage and route of administration
It can be given as a 500 mg LD followed by 250 mg every 2 weeks, subcutaneous route OR 500 mg LD, followed by 250 mg every 4 weeks.
It can also be given as a 250 mg LD, followed by 125 mg every 4 weeks.[18]
Anti-IL-31 agents
Nemolizumab
IL-31 is one of the proinflammatory cytokines that has an important role in mediating pruritus by overexpressing IL-31 receptors on sensory nerves[19,20] and is well known to accentuate and maintain the itch-scratch cycle that results in disruption of the skin barrier in AD.[21]
Mechanism of action
Nemolizumab is a humanized anti-IL-31 Ra monoclonal antibody of class IgG2k that antagonizes IL-31 by binding to its receptor.
At present, there are three ongoing clinical Phase 3 trials on nemolizumab safety and efficacy among patients suffering from AD (NCT03989349, NCT03989206, NCT03985943, ClinicalTrials.gov).[19-22]
ISB 830
ISB 830 (previously GBR 830) is a humanized IgG1 anti-OX40 monoclonal antibody. Binding of OX40 with its ligand (OX40L) which is expressed on antigen presenting cells accentuates effector T-cell responses. Guttman-Yassky et al. investigated the efficacy and safety of GBR830 in an Phase-2a and placebo-controlled study among patients having moderate-to-severe AD.[23]
Results showed that ISB830 was well tolerated and showed a significant reduction in Th1-, Th2, and Th17/22 expression in lesional skin compared to placebo. Furthermore, a significant reduction in the epidermal thickness was observed.
Anti TSLP agent
Tezepelumab
Tezepelumab is a monoclonal antibody, targeting TSLP.
In a Phase-2 study, 113 patients were randomized at a 1:1 ratio and treated either with placebo or subcutaneous tezepelumab every 2 weeks.
The results of the trial showed that a higher percentage of patients treated with tezepelumab reached an eczema area severity index-50 (EASI-50) after 12 weeks compared to the placebo group; however, it was not statistically significant (P = 0.91).[24,25]
Anti-IL-22 agents
Fezakinumab
Th-22 cells play an important role in pathomechanism of AD. Th-22 cells express IL-22, which activates a receptor that causes epidermal hyperplasia, migration of keratinocytes, downregulation of keratinocyte differentiation, and elevation of pro-inflammatory cytokines.
Guttmann-Yasky et al. performed a randomized, double-blind, and placebo-controlled trial to test the efficacy and safety of Fezakinumab, a monoclonal IgG-antibody against IL-22. A significant reduction in the SCORAD (≥50) was found in the subgroup of patients with severe AD compared to placebo (P < 0.029), but this effect was not achieved by the entire study population.[26]
Anti-IgE
Omalizumab and Ligelizumab
Mechanism of action
These drugs prevent the interaction of IgE with its receptors by binding to the Fc portion (CH3 domain).
Binding of omalizumab to free IgE blocks its binding to its receptors, thus blunting allergen induced mediator release. Moreover, IgE neutralization decreases serum expression of cytokines such as IL-5, IL8, IL13, and negatively regulates recruitment of immune cells.
Dosage and route of administration
It is given in dosage of 150-300 mg subcutaneously at an interval of 2-4 weeks for 3-6 months.
In a systematic review on patients with AD on Omalizumab, less than half patients having AD, and additionally displayed significant clinical improvement.[27]
A Phase 4 clinical trial by Chan et al. on pediatric population showed efficacy of omalizumab in the treatment of AD and also reduced the need for topical steroid use.[28,29]
JAK inhibitors in AD
The JAK-STAT pathway triggers multiple cytokines inducing cutaneous inflammation in AD. JAK inhibitors target specific receptor-associated kinases, thereby preventing the inflammatory cascade.[30,31]
Several JAK inhibitors in oral and topical formulations targeting different JAK receptors represent potential therapeutic options for AD.
The JAK inhibitors that have been approved by the United States FDA (USFDA) are discussed below.
Approved systemic JAK inhibitors
Abrocitinib
Abrocitinib is a recently FDA approved oral JAK1 inhibitor for patients with moderate to severe refractory AD. Previous approved age group was >18 years of age that has now been expanded to include adolescents from 12- 18 years of age group.
During phase IIb & phase III of clinical trials, abrocitinib was found to be efficacious in 47.5% patients on 200 mg abrocitinib and 32.0% on 100 mg abrocitinib.
The Investigator Global Assessment (IGA) of 0 or 1 at 12 weeks adolescents (aged 12- to 17-year olds) suffering from moderate-to-severe AD, three phase three clinical studies (JADE TEEN [NCT03796676]; JADE MONO-1 [NCT03349060]; JADE MONO-2 [NCT03575871] found abrocitinib as monotherapy or as combination therapy to be effective in improving the clinical condition.[32]
Approved dose - 100mg, 200mg OD Adverse effects noted were upper respiratory tract infection, herpes simplex, herpes zoster, nausea, vomiting, dizziness & thrombocytopenia.[33-37]
Baricitinib
Apart from dupilumab, another “first line” systemic therapy, baricitinib, has been approved. On September 18, 2020, the EMA granted an extension of the marketing authorization for baricitinib for the treatment of adult patients with moderate to severe AD.[38] The efficacy of Baricitinib for the treatment of moderate-to-severe AD was demonstrated, in a Phase II study of baricitinib in combination with topical steroids. After 16 weeks, based on an EASI-50, results were significant with 4 mg baricitinib daily (61%) compared to the placebo group (37%).[39] Two independent, multicenter, and double-blind Phase III studies (BREEZE-AD1 and BREEZE-AD2) were conducted, and were published in January 2020.[40] Patients on 4 mg or 2mg daily reached the primary endpoint than in the placebo group (for 4 mg dosing in BREEZE-AD1 16.8% placebo: 4.8%; and in BREEZE-AD2 13.8%, versus placebo: 4.5%).[41] Approved dose- 4 mg OD, 2 mg for >75 years of age.
The route of administration of baricitinib is per-oral. The adverse effects noted were upper respiratory tract infection and herpes simplex infection.
Upadacitinib
Upadacitinib is an oral JAK 1 Inhibitor recently approved by FDA for adolescents of >12 years and adults having moderate to severe refractory AD.
Approved dose - 15mg, 30mg OD
In clinical trial Measure UP-1 and -2 monotherapy for Upadacitinib used as a monotherapy taken daily, after 16 weeks on either 15mg or 30mg results for Investigator Global Assessment (IGA) showed 48.1% and 38.8% of patients (15mg dose); 62% and 52% of patients (30mg dose) had an IGA score of clear to almost clear (IGA 0 or 1) compared to 8.4% and 4.7% on placebo, and Eczema Area and Severity Index (EASI)-75 showed 69.6% and 60.1% of patients (15mg dose); 79.7% and 72.9% of patients (30mg dose) achieved an EASI-75 compared to 16.3% and 13.3% on placebo.[42-44]
Adverse events - Mild to Moderate Acne.
Approved topical JAK inhibitors
Delgocitinib
Delgocitinib initially known as JTE-052, is a JAK inhibitor on JAK1, JAK2, JAK3, and TYK2, In a double-blind, vehicle-controlled Phase III study, delgocitinib 0.5% ointment was evaluated in patients with moderate-to-severe AD. Primary endpoint was the change in modified EASI (mEASI) after 4 weeks; mEASI score is 0–92 in which 0 is clear, 1–8.9 mild, 9–29.9 moderate, and 30.0–90 severe.
The reduction in mEASI was significantly more in the delgocitinib group (−44.3% vs. 1.7%). Furthermore, 51.9% achieved an at least 50% improvement in mEASI -50 with delgocitinib compared with 11.5% in the vehicle group. In January 2020, delgocitinib received approval in Japan as a 0.5% ointment (Corectim), making it the first approved topical JAK inhibitor worldwide.[45]
Ruxolitinib
The FDA approval was based on data from the Topical Ruxolitinib Evaluation in AD (TRuE-AD) clinical trial program, consisting of two randomized, double-blind, and vehicle-controlled Phase 3 studies (TRuE-AD1 and TRuE-AD 2)[49-52] evaluating the safety and efficacy of the cream in more than 1200 adolescents and adults with mild-to-moderate AD.
Results were significant with medicated cream 1.5% twice daily (BID), compared with non-medicated cream.
In addition, significantly more patients treated with ruxolitinib cream achieved Investigator’s global assessment Treatment success (IGA-TS; primary end point) at week 8: 53.8% in TRuE-AD1 and 51.3% in TRuE-AD2, compared with nonmedicated treatment (15.1% in TRuE-AD1, 7.6% in TRuE-AD2; P < 0.0001). The other JAK inhibitors in trial phase and their formulations are summarized in Table 2.[53-56]
Phosphodiesterase inhibitors
Phosphodiesterase 4 is important in the pathogenesis of AD. Crisaborole is an inhibitor of this enzyme. A 2% ointment of crisaborole, a boron compound, has been approved for the treatment of mild-to-moderate AD in patients above 2 years of age by the USFDA. This drug still awaits approval for use in India.[57,58]
To date, there have been no randomized, double-blind comparative clinical trials conducted in Indian patients with AD. Clinical trials and studies done in other countries have shown a reasonable efficacy and safety profile, and also a substitute for topical steroids in the treatment of mild to moderate AD.[58]
CONCLUSION
With the advancement of clinical research, a deeper understanding of the pathomechanism and responsive therapies has been realized in the treatment of AD. Promising results have been achieved with crisaborole, dupilumab, and abrocitinib, which have been approved by the FDA for use in AD. Among newer modalities of treatment JAK inhibitors are slowly gaining approval for AD, such as delgocitinib and ruxolitinib. Many other biologic agents are still in various phases of trial. Although deemed expensive, these drugs may be an elixir in improving the quality of life and providing a positive psychosocial impact achieved by faster, safer, and long-lasting remissions.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284-93.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic treatments in the management of atopic dermatitis: A systematic review and meta-analysis. Allergy. 2021;76:1053-76.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic treatment of adult atopic dermatitis: A review. Dermatol Ther (Heidelb). 2017;7:1-23.
- [CrossRef] [PubMed] [Google Scholar]
- Crisaborole 2% ointment (eucrisa) for atopic dermatitis. Skin Ther Lett. 2019;24:4-6.
- [Google Scholar]
- Dupilumab: A review of present indications and off-label uses. J Investig Allergol Clin Immunol. 2022;32:97-115.
- [CrossRef] [PubMed] [Google Scholar]
- Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-60.
- [CrossRef] [PubMed] [Google Scholar]
- New treatments in atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126:21-31.
- [CrossRef] [PubMed] [Google Scholar]
- Itch: From mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-90.
- [CrossRef] [PubMed] [Google Scholar]
- Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-28.e13.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis treatment: Current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab: A review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 Suppl 1):S28-36.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab use in dermatologic conditions beyond atopic dermatitis-a systematic review. J Dermatol Treat. 2021;32:19-28.
- [CrossRef] [PubMed] [Google Scholar]
- Dupixent (Dupilumab) Prescribing Information. Tarrytown, New York: Regeneron Pharmaceuticals; 2017.
- [Google Scholar]
- Sanofi.com. Available from: https://www.sanofi.com/en/media-room/press-releases/2020/2020-05-26 [Last accessed on 2023 May 26]
- [Google Scholar]
- Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75:54-62.
- [CrossRef] [PubMed] [Google Scholar]
- Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184:437-49.
- [CrossRef] [PubMed] [Google Scholar]
- Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-63.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic potential of lebrikizumab in the treatment of atopic dermatitis. J Asthma Allergy. 2020;13:109-14.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145:173-82.
- [CrossRef] [PubMed] [Google Scholar]
- The pruritus-and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-8.e24.
- [CrossRef] [PubMed] [Google Scholar]
- Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383:141-50.
- [CrossRef] [PubMed] [Google Scholar]
- Nemolizumab for atopic dermatitis. Drugs Today (Barc). 2022;58:159-73.
- [CrossRef] [PubMed] [Google Scholar]
- GBR830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:482-93.
- [CrossRef] [PubMed] [Google Scholar]
- The New Era of biologics in atopic dermatitis: A review. Dermatol Pract Concept. 2021;11:e2021144.
- [CrossRef] [PubMed] [Google Scholar]
- Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80:1013-21.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J Am Acad Dermatol. 2018;78:872-81.e6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of omalizumab in patients with atopic dermatitis: A systematic review and meta-analysis. J Allergy Clin Immunol. 2016;138:1719-22.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment effect of omalizumab on severe pediatric atopic dermatitis: The ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- [CrossRef] [PubMed] [Google Scholar]
- Biologics for treatment of atopic dermatitis: Current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-65.
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- [CrossRef] [PubMed] [Google Scholar]
- Modern therapies in atopic dermatitis: Biologics and small molecule drugs. J Dtsch Dermatol Ges. 2020;18:1085-92.
- [CrossRef] [Google Scholar]
- Impact of oral abrocitinib on signs, symptoms and quality of life among adolescents with moderate-to-severe atopic dermatitis: An analysis of patient-reported outcomes. J Eur Acad Dermatol Venereol. 2021;36:422-33.
- [CrossRef] [PubMed] [Google Scholar]
- Once-daily abrocitinib for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents aged 12 years and over: A short review of current clinical perspectives. Ther Clin Risk Manag. 2022;18:399-407.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: A phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-66.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156:863-73.
- [CrossRef] [PubMed] [Google Scholar]
- Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-12.
- [CrossRef] [PubMed] [Google Scholar]
- New Oral Treatment for Moderate to Severe Atopic Dermatitis. 2020. Netherlands: European Medicines Agency; Available from: https://www.ema.europa.eu/en/news/new-oral-treatment-moderate-severe-atopic-dermatitis [Last accessed on 2023 May 26]
- [Google Scholar]
- Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-55.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 Phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-52.
- [CrossRef] [PubMed] [Google Scholar]
- Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35:476-85.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of upadacitinib treatment in adolescents with moderate-to-severe atopic dermatitis: Analysis of the measure up 1, measure up 2, and ad up randomized clinical trials. JAMA Dermatol. 2023;159:526-35.
- [CrossRef] [Google Scholar]
- Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-68.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-81.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis. J Dermatol. 2018;45:701-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: A phase II, multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol. 2018;178:424-32.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575-83.
- [CrossRef] [PubMed] [Google Scholar]
- Delgocitinib ointment, topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-31.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145:572-82.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: Results from a phase 2, randomized, dose-ranging, vehicle-and active-controlled study. J Am Acad Dermatol. 2020;82:1305-13.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-72.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Ruxolitinib Evaluation in Atopic Dermatitis Study 1 (TRuE AD1)-an Efficacy and Safety Study of Ruxolitinib Cream in Adolescents and Adults with Atopic Dermatitis. Clinicaltrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03745638 [Last accessed on 2023 May 26]
- [Google Scholar]
- Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br J Dermatol. 2016;175:902-11.
- [CrossRef] [PubMed] [Google Scholar]
- A phase 1b, randomized, single-center trial of topical cerdulatinib (DMVT-502) in patients with mild-to-moderate atopic dermatitis. J Invest Dermatol. 2021;141:1847-51.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-9.
- [CrossRef] [PubMed] [Google Scholar]
- The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: Results from a randomized double-blind placebo-controlled study. Br J Dermatol. 2019;181:733-42.
- [CrossRef] [PubMed] [Google Scholar]
- Crisaborole for the treatment of atopic dermatitis in Indian patients: An evidence-based consensus statement. Indian J Drug Dermatol. 2021;7:7-11.
- [CrossRef] [Google Scholar]
- Topical agents for the treatment of atopic dermatitis. J Drugs Dermatol. 2020;19:50-64.
- [CrossRef] [PubMed] [Google Scholar]







