Translate this page into:
Patch testing with Indian standard series of 20 allergens in 125 patients of chronic dermatitis and dermatoses
*Corresponding author: Balasaraswathy Panambur, Department of Dermatology, Spandana Centre for Metabolic Medicine, Mangaluru, Karnataka, India. dr.balasaraswathy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Panambur B. Patch testing with Indian standard series of 20 allergens in 125 patients of chronic dermatitis and dermatoses. Indian J Skin Allergy. 2024;3:111-8. doi: 10.25259/IJSA_16_2024
Abstract
Objectives:
Patch testing is generally done in cases of allergic contact dermatitis to find the offending allergen/s. It has been less reported in patients clinically presenting as non-eczematous contact reactions such as lichen planus, lichenoid eruption, lichen planus pigmentosus and facial melanosis overlap. In addition, it may give clues to the sensitizers leading to chronic, recurring dermatitis and overlap of dermatoses in the form of systemic contact dermatitis. The aim of the present study was to identify the sensitizer/s with patch testing in patients presenting with non-eczematous contact reactions as well as chronic, relapsing dermatitis and overlap of dermatoses.
Material and Methods:
In the present study, patch testing was done using the Indian standard series in 125 cases presenting clinically as lichen planus, lichen planus pigmentosus, facial melanosis, cheilitis, mucosal lichen planus and vulvar dermatitis, in addition to cases of chronic and recurrent dermatitis such as nummular eczema, lichenoid eruption, pompholyx, photo aggravated dermatitis, air borne contact and hand and feet dermatitis.
Results:
The tests were positive in 102 (81.6%) patients, negative in 16, doubtful in 3 and invalid in 4 patients. Positive results were found in 100% cases of photo aggravated dermatitis and chronic dermatitis of hands/feet dermatitis and 76% cases of facial melanosis and lichen planus pigmentosus overlap, 88.9% cases of nummular eczema, 81.8% cases of lichenoid eruption, 75% cases of lichen planus, 57% cases of atopic eczema, 66.6% cases of pompholyx, and 100% of mucosal lichen planus. Test for nickel was positive in 40 (32%) cases, parthenium in 37 (29.6%), cobalt in 23 (18.4%), fragrance in 19 (15.2%), paraphenylenediamine in 17 (13.6%), paraben in 9 (7.2%) and neomycin in 5 (4%) cases, either alone or in combination. In the present study, in addition to eczematous dermatoses, non-eczematous conditions such as lichen planus, lichenoid eruption, lichen planus pigmentosus and facial melanosis also showed positive results on patch testing.
Conclusion:
Patch testing should be considered in patients presenting with chronic and recurrent dermatitis and overlap of more than one type of dermatoses. Identification and elimination of culprit allergens can help in achieving long term remission in these chronic conditions.
Keywords
Chronic dermatitis
Non-eczematous dermatitis
Systemic contact dermatitis
Patch testing
Dietary intervention
INTRODUCTION
Allergic contact dermatitis is a common cutaneous eczematous disorder caused by a range of environmental substances.[1] Non-eczematous forms of contact dermatitis like erythema, multiforme-like contact dermatitis, lichenoid contact dermatitis, pigmented contact dermatitis, pustular contact dermatitis, and dyshidrosiform contact dermatitis are also known and may even be more common than the classic eczematous forms.[1] These non-eczematous forms of contact dermatitis are more often dependent on systemic administration of the allergens.[1] Systemic contact dermatitis (SCD) is defined as the provocation or exacerbation of dermatitis by non-cutaneous exposure to an allergen in a person who is already sensitized through the cutaneous route.[2] SCD is poorly understood, and epidemiological data for SCD in the general population are scarce.[2] It is therefore necessary to consider the possibility of SCD in cutaneous disorders presenting chronically, variedly, and responding poorly to conventional treatment.
Patch testing is carried out to find the offending allergen/s causing a delayed type of hypersensitivity in patients with allergic contact dermatitis. It not only helps in confirming contact sensitivity suspected from clinical history but also aids in unveiling unsuspected sensitivities.[3] Patch testing will also give information on occult or superimposed allergens in patients with recurrent or non-responsive conditions.[4] In addition, it can give clues to sensitizers causing nummular eczema (NE), pompholyx or dyshidrotic eczema, chronic dermatitis of hands and feet, generalized maculopapularvesicular rash, baboon syndrome, disseminated patchy dermatitis, vulvar pruritus/dermatitis, pruritus ani, cheilitis, lichen planus (LP) of mucosa, amongst others, presenting as SCD.[2,5] Patch testing has been less reported in patients clinically presenting as non-eczematous contact reactions such as LP, lichenoid eruption (LE), LP pigmentosus (LPP), and facial melanosis (FM)/pigmented contact dermatitis (PCD). The present study aimed to identify the sensitizer/s with patch testing in patients presenting with chronic, relapsing dermatitis and overlap of dermatoses.
MATERIAL AND METHODS
Patch testing was done, after due consent, in 125 consecutive patients presenting with chronic relapsing dermatitis and dermatoses. The inclusion criteria were chronic recurrent dermatitis and dermatoses with overlapping features, atopics with chronic dermatitis lasting months to years, and failure to achieve satisfactory response to standard treatment. The exclusion criteria were patients on anti-inflammatory and immunosuppression medications and patients with acute generalized dermatitis.
The diagnosis was based on typical clinical features, with biopsy being done in 4 cases of LPP. NE was diagnosed based on the clinical presentation of oval-to-round plaques studded with vesicles, occurring bilaterally, predominantly on the extremities. LE was diagnosed based on shiny violaceous papules and plaques on both flexor and extensor surfaces, without mucosal involvement. Photoaggravated dermatitis (PAD) was considered based on a diffuse scaly erythematous rash on the face, neck, and exposed parts of the forearms, typically sparing the skin around the eyes. FM and LPP overlap was considered in the presence of diffuse blue-gray hyperpigmentation of the face with oval-shaped gray-brown patches on the neck and arms. Detailed relevant history regarding the duration of the eruption and of allergies and atopy in patients and their families and of exposure to known allergens were obtained. Relevance was determined by history of past exposure to known allergens leading to dermatitis, like ear lobe eczema on using metal jewelry, rash following use of hair dye, occupation leading to exposure to certain allergens, and family history of dermatitis to such allergens. In some cases, relevance was confirmed post-testing revelation by patients and based on improvement on elimination of allergen/s.
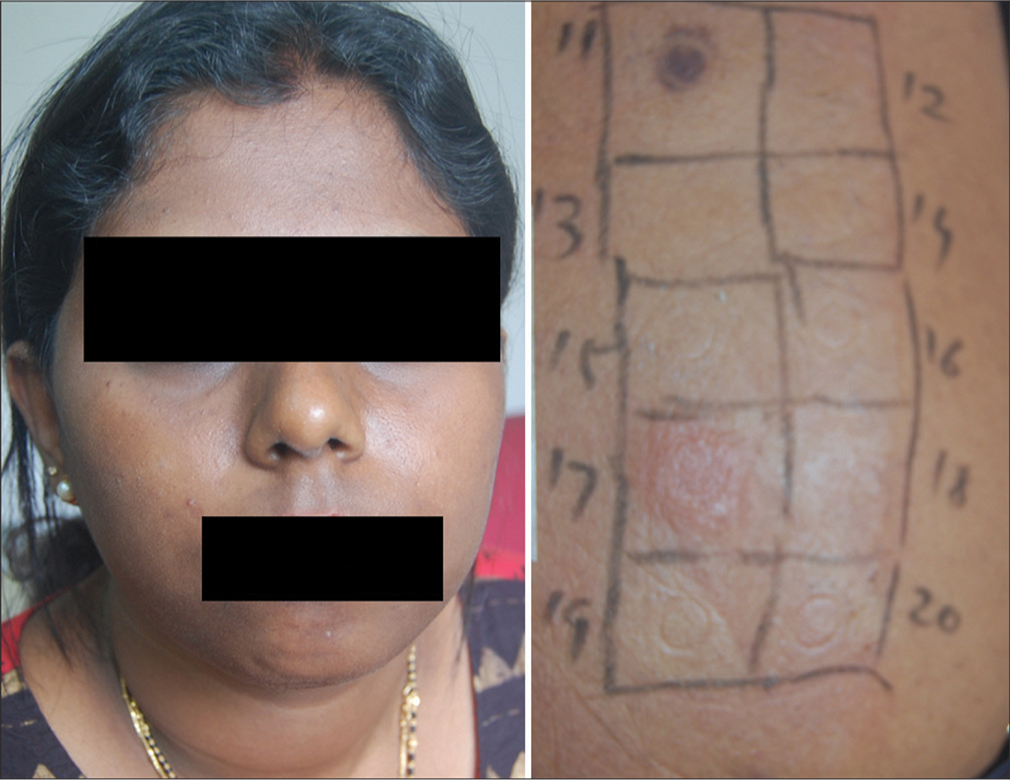
In all patients, patch testing was done after stopping the anti-inflammatory and anti-histaminic medications for more than 4 weeks, and the skin on the back was free of lesions. Patch testing was done with the Indian Standard series, comprising 20 allergens [Table 1], standardized by CODFI and manufactured by Credisol®, Navi Mumbai, India, distributed by Systopic Laboratories, New Delhi, India. The patches were applied on the upper back and the readings were done after 48 h (day 2) and 72 h (day 3), according to the recommendations of the International Contact Dermatitis Research Group (ICDRG). The results were graded as negative, doubtful (?+), mild reaction, possible erythema, infiltration and papules (+), strong reaction, erythema, infiltration, papules and vesicles (++), and very strong reaction, intense erythema, infiltration and coalescing vesicles (+++) (ICDRG). Lesions and patch test results were photographed in all patients.
| S. No. | Allergen | Strength (%) | Vehicle |
|---|---|---|---|
| 1. | Vaseline | 100 | |
| 2. | Balsam of peru | 10 | Petrolatum |
| 3. | Formaldehyde | 1 | Aqueous |
| 4. | Mercaptobenzothiazole | 1 | Petrolatum |
| 5. | Potassium bichromate | 0.5 | Petrolatum |
| 6. | Nickel sulfate hexahydrate | 5 | Aqueous |
| 7. | Cobalt sulfate | 5 | Aqueous |
| 8. | Colophony | 10 | Petrolatum |
| 9. | Epoxy resins | 1 | Petrolatum |
| 10. | Paraben mix | 9 | Petrolatum |
| 11. | PPD | 5 | Petrolatum |
| 12. | Parthenium | 15 | Petrolatum |
| 13. | Neomycin sulfate | 20 | Petrolatum |
| 14. | Benzocaine | 5 | Petrolatum |
| 15. | Wool alcohol | 30 | Petrolatum |
| 16. | Chlorcresol | 1 | Petrolatum |
| 17. | Fragrance mix | 8 | Petrolatum |
| 18. | Thiuram mix | 1 | Petrolatum |
| 19. | Nitrofurazone | 1 | Petrolatum |
| 20. | Black rubber mix pet | 0.6 | Petrolatum |
PPD: Paraphenylenediamine
RESULTS
The patients belonged to the age range of 4–70 years, 97 (77.6%) being females and 28 males [Table 2]. The patch test was positive in 102 (81.6%) patients, negative in 16, doubtful in 3, and unsuccessful in 4 patients [Table 2]. Of the positive cases, 52 (51%) had positive results for a single allergen and 50 (49%) for more than one allergen [Table 3]. Atopic eczema (AE), pompholyx, and NE were more common in younger age group (6-54 years) and females. All patients in the younger age group had a history of atopy/atopic diathesis. LE, with and without NE, and hand and feet dermatitis were seen in later age group (19–65 years), with/without atopy. FM with/without LPP was seen in the 25– 60-year age group, with female preponderance (17:4). LPP was also more common in females than males (7:2). Of the 28 male cases, 22 tested positive, 6 of them had PAD, 4 had hand dermatitis, 4 had FM and LPP, and the remaining had LE, NE, and atopiform eczema, either alone or as overlap. Males presenting with hand dermatitis had their occupations as contributing factors.
| Diagnosis | Total cases | Age in years | Female (Positive) | Male (Positive) | Total positive (Percent) |
|---|---|---|---|---|---|
| NE | 18 | 7–54 | 15 (13) | 3 (3) | 16 (89) |
| NE+LE | 5 | 19–65 | 4 (4) | 1 (1) | 5 (100) |
| NE+AD/Atopiform/Pom | 3 | 13–54 | 1 (1) | 2 (2) | 3 (100) |
| FM | 6 | 26–60 | 4 (3) | 2 (1) | 4 (67) |
| FM+LPP | 11 | 25–65 | 9 (8) | 2 (2) | 10 (91) |
| FM+LE/CD-F/PLE | 4 | 26–53 | 4 (4) | 0 | 4 (100) |
| LPP | 8 | 11–45 | 6 (4) | 2 (1) | 5 (63) |
| LPP+PLE | 1 | 51 | 1 (1) | 0 | 1 (100) |
| LE | 11 | 21–51 | 10 (8) | 1 (1) | 9 (82) |
| LE+CD-H/Chr Urt | 2 | 40–64 | 1 (1) | 1 (1) | 2 (100) |
| PAD | 9 | 22–70 | 5 (5) | 4 (4) | 9 (100) |
| PAD+FM/LPP/LE | 4 | 32–68 | 2 (1) | 2 (2) | 3 (75) |
| LP/MLP | 10 | 10–68 | 9 (7) | 1 (1) | 8 (80) |
| CD-H/F | 7 | 14–51 | 4 (4) | 3 (3) | 7 (100) |
| AE | 7 | 4–14 | 7 (4) | 0 | 4 (57% |
| ABCD/+PLE/Urt/AD | 4 | 5–47 | 3 (3) | 1 (1) | 4 (100) |
| Pom | 3 | 6–28 | 3 (2) | 0 | 2 (67) |
| Others: ACD, Chronic eczema, GVE, LN, POD, PLE, Pruritus, Stasis eczema, VD | 12 | 3–62 | 9 (5) | 3 (0) | 5 (42) |
| Total | 125 | 4–70 | 97 | 28 | 102 (82) |
ABCD: Airborne contact dermatitis, ACD: Allergic contact dermatitis, AE: Atopic eczema, CD: Chronic dermatitis, H: Hands, F: Feet, Chr Urt: Chronic urticaria, FM: Facial melanosis, GVE: Generalized vesicular eczema, LE: Lichenoid eruption, LN: Lichen nitidis, LP: Lichen planus, LPP: Lichen planus pigmentosus, MLP: Mucosal lichen planus, NE: Nummular eczema, PAD: Photoaggravated dermatitis, PLE: Polymorphic light eruption, POD: Perioral dermatitis, Pom: Pompholyx, VD: Vulvar dermatitis
| Allergen | Number positive | Clinical conditions | ||
|---|---|---|---|---|
| Total (%) | Alone | Combination | ||
| Nickel sulfate hexahydrate 5% Aq | 40 (32) | 18 | 22 | LPP – 4, FM – 2, FM+LPP – 5, FM+LE – 2, LE – 6, LE+NE – 3, NE – 5, LP – 2, LPP+PLE – 1, NE+Pom – 1, MLP – 2; PAD, Atopiform, CD-HF, CD-F, GVE, VD, POD – 1 each |
| Parthenium 15% Pet | 37 (29.6) | 13 | 24 | PAD – 6, NE – 3, NE+AE – 1, NE+atopiform – 1, FM+LPP – 3, LPP – 1, FM+PAD – 1, LPP+PAD – 1, LE – 4, LP – 3, AE- 3, Pom – 1, Pom+AE – 1, PLE – 1, MLP – 2, VD – 1, ABCD+AE – 1, CD-HF – 1, CD-H+LE – 1 |
| Cobalt sulfate 5% Aq | 23 (18.4) | 3 | 20 | NE – 5, NE+LE – 2, NE+Pom – 1, POM+AE – 1, LE – 4, PAD – 2, PAD+LPP – 1, FM – 1, FP+LPP – 1, LP - 1, ABCD+Urt/PLE – 2, MLP – 1, CD-H – 1 |
| Fragrance mix 8% Pet | 19 (15.2) | 6 | 13 | FM, FM+LPP, FM+PAD, FM+PLE, LPP+PLE – 7; PAD – 3, NE- 3; LE, LE/NE – 3, LP -1, CD-F – 2 |
| PPD 5% Pet | 17 (13.6) | 3 | 14 | FM/LPP -7, CD-H/F – 4, PAD/LE – 4, NE – 1, ACD – 1 |
| Paraben mix 9% Pet | 9 (7.2) | 2 | 7 | NE – 3; FM, FM+PAD, LE, LP, Pom+NE, CD-H -1 each |
| Neomycin sulfate 20% Pet | 5 (4) | 2 | 3 | FM+LPP, NE, GVE, CH-D, VD – 1 each |
| Formaldehyde Aq | 8 (6.4) | 1 | 7 | ABCD – 1, PAD -2, NE – 2; FM, FM+LE, FM+LPP – 1 each |
| Epoxy resins 1% Pet | 6 (4.8) | 1 | 5 | LE- 2, NE – 2, Chr Urt – 1, LP – 1 |
| Benzocaine 5% Pet | 5 (4) | 0 | 5 | PAD, LE, FM+CD-F, CD-HF, Pom+AE – 1 each |
| Wool alcohol 30% Pet | 2 (1.6) | 0 | 2 | NE, ABCD+PLE – 1 each |
| Chlorcresol 1% Pet | 2 (1.6) | 0 | 2 | LE, NE – 1 each |
| Nitrofurazone 1% Pet | 1 (0.8) | 0 | 1 | LE – 1 |
| Mercaptobenzothiazole 1% Pet | 1 (0.8) | 0 | 1 | LE+NE – 1 |
| Potassium bichromate 0.5% Pet | 2 (1.6) | 0 | 2 | LE+NE - 1, NE – 1 |
| Thiuram mix 1% Pet | 3 (2.4 | 2 | 1 | LE, NE, NE+Pom – 1 each |
| Black rubber mix 0.6% Pet | 2 (1.6) | 1 | 1 | LE, CD-HF – 1 each |
| Colophony 10% Pet | 3 (2.4) | 0 | 3 | PAD, LE+NE, AE – 1 each |
Aq: Aqueous, Pet: In petrolatum vehicle, ABCD: Airborne contact dermatitis, ACD: Allergic contact dermatitis, AE: Atopic eczema, CD: Chronic dermatitis, H: Hands, F: Feet, Chr Urt: Chronic urticaria, FM: Facial melanosis, GVE: Generalized vesicular eczema, LE-Lichenoid eruption, LP: Lichen planus, LPP: Lichen planus pigmentosus, MLP: Mucosal lichen planus, NE: Nummular eczema, PAD: Photoaggravated dermatitis, PLE: Polymorphic light eruption, POD: Perioral dermatitis, Pom: pompholyx, VD: Vulvar dermatitis, PPD: Paraphenylenediamine
Nineteen (76%) of the 25 cases of FM/LPP, 16 of 18 (88.9%) cases of NE, 9 of 11 (81.8%) cases of LE, all 9 (100%) cases of PAD, 6 of 8 (75%) cases of LP, all 5 (100%) cases of chronic dermatitis of hands/feet, 2 of 3 cases of pompholyx, 4 of 7 cases of AE, both cases of mucosal LP, and all tested cases of atopiform (pseudo atopic) eczema, airborne contact dermatitis (ABCD), allergic contact dermatitis, and vulvar dermatitis showed positive results [Table 2].
Test for nickel was positive in 40 (32%) cases, parthenium in 37 (29.6%), cobalt in 23 (18.4%), fragrance mix in 19 (15.2%), paraphenylenediamine (PPD) in 17 (13.6%), parabens in 9 (7.2%), formaldehyde in 8 (6.4%), epoxy resins in 6 (4.8%), neomycin in 5 (4%), thiuram in 3 (2.4%), black rubber mix in 2 (0.6%) cases, alone or in combination with other allergens [Table 3]. Other allergens were positive in combination in smaller numbers [Table 3].
Of the 40 cases testing positive for nickel, 38 were females and 2 males, aged between 3 and 56 years. Eighteen cases were positive for nickel alone, and 22 had positive tests for nickel along with other allergens. Five of the nickel-positive cases had FM with LPP, 4 had LPP, 2 had FM, 2 had FM with LE, 1 had LPP with PLE, 6 had LE, 5 had NE, 3 had NE with LE, 1 had NE and pompholyx, 2 had LP, and the remaining 9 cases had atopiform eczema, chronic dermatitis of hand/feet, generalized vesicular eczema, mucosal LP, PAD, perioral dermatitis, and vulvar dermatitis [Figures 1-4; Table 3]. Test for cobalt was positive in 23 cases, 20 of them with other allergens. Of the cobalt-positive cases, 20 were females, and 3 were males, aged between 9 and 65 years. Five of the cobalt-positive cases had NE, 4 had LE, 2 had NE with LE, 2 had PAD, and the remaining cases had pompholyx, FM, LPP, PAD, LP, chronic hand dermatitis, mucosal LP with cheilitis, and ABCD with PLE/urticaria [Table 3]. Therefore, nickel and cobalt showed positivity in varied skin conditions.
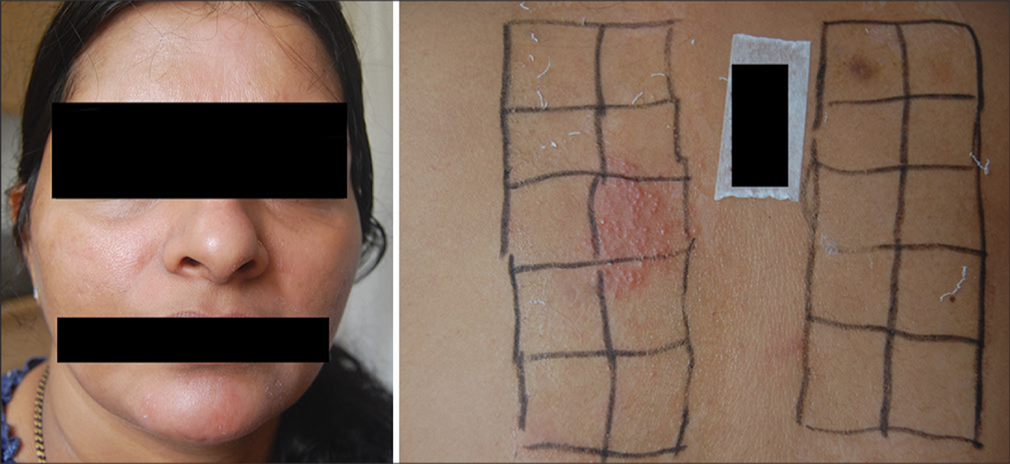
- Photoaggravated dermatitis showing positive test for nickel and parthenium.
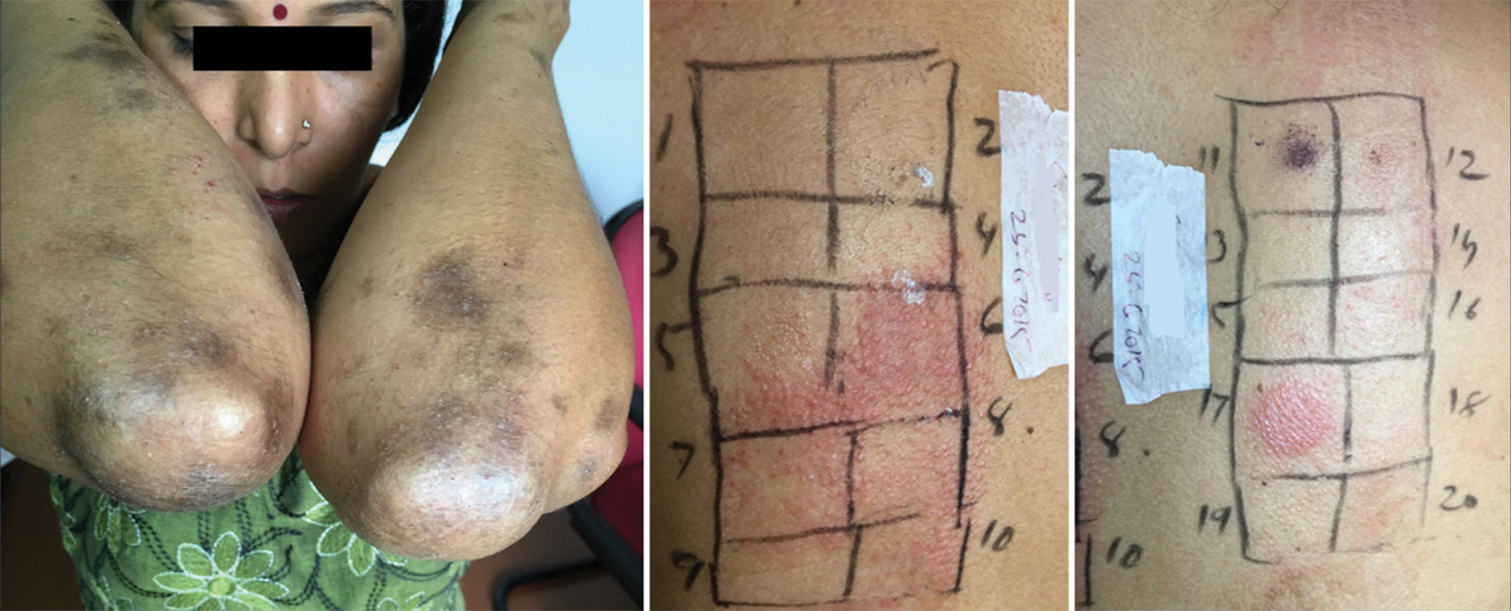
- Lichenoid eruption showing positive test for nickel and fragrance.
- Lichen planus pigmentosus showing positive test for nickel.
- Lichenoid eruption positive to nickel.
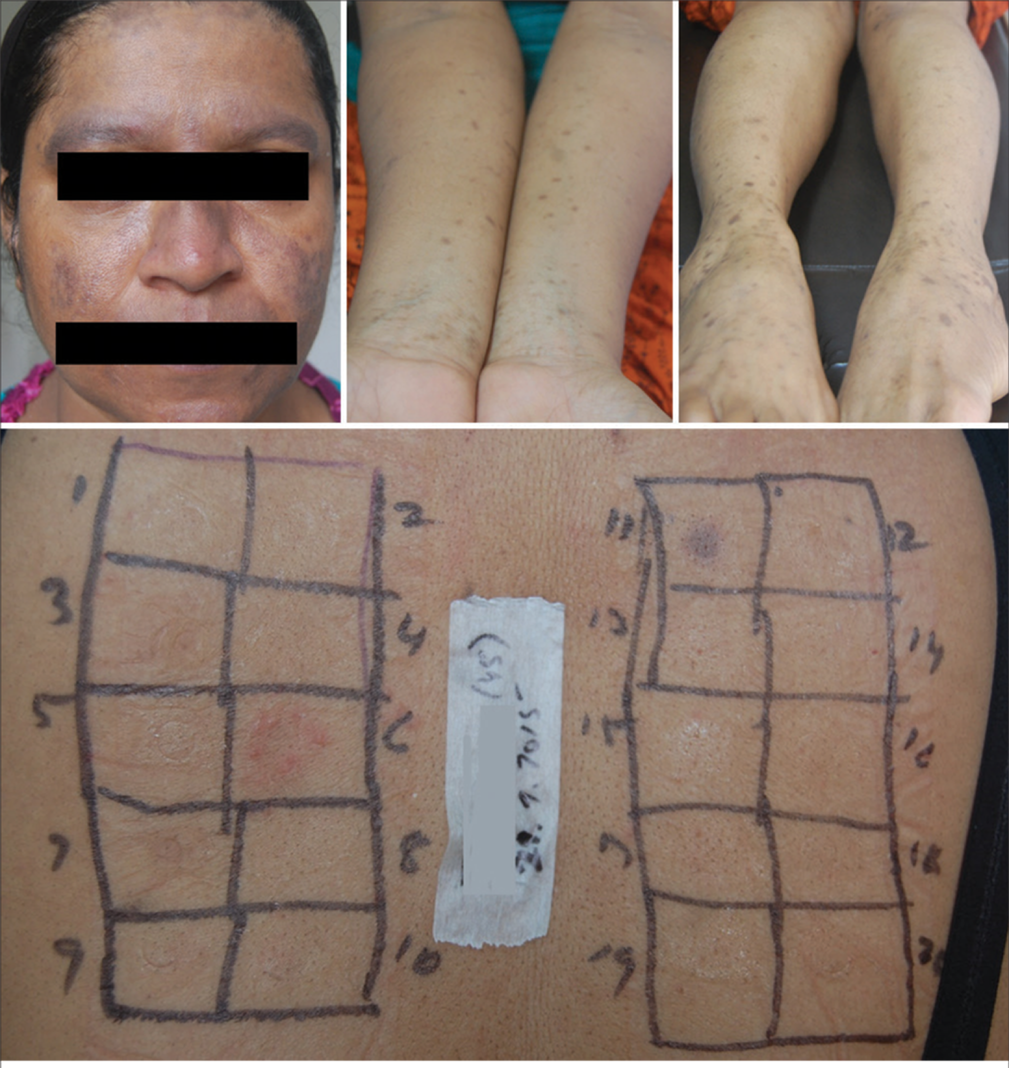
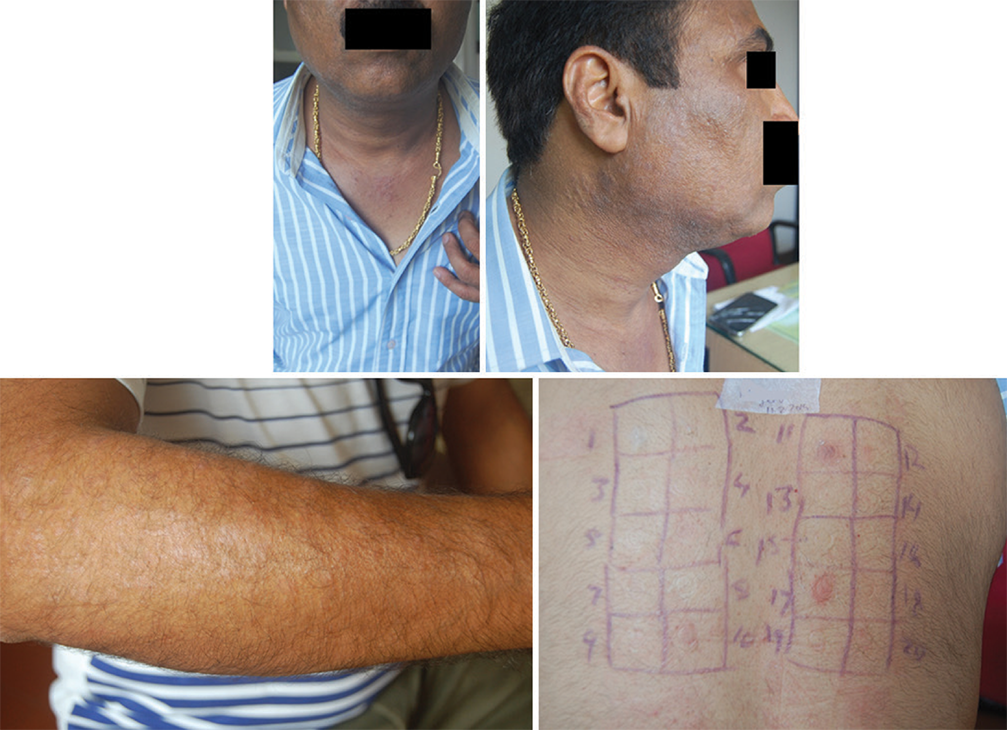
All patients found sensitive to nickel were advised to eliminate food items high in nickel, such as cocoa (chocolates), nuts, soy, oats, millets, tubers, leafy vegetables, pineapple, dates, figs, shelled fish, and canned foods. Cobalt sensitive patients were advised to avoid dairy products in addition. All these patients showed significant remission of their skin lesions within 6 weeks and maintained the remission on maintenance of dietary elimination. However, patients admitted to reappearance of lesions, though less severe, on consumption of culprit foods [Figures 5-7].
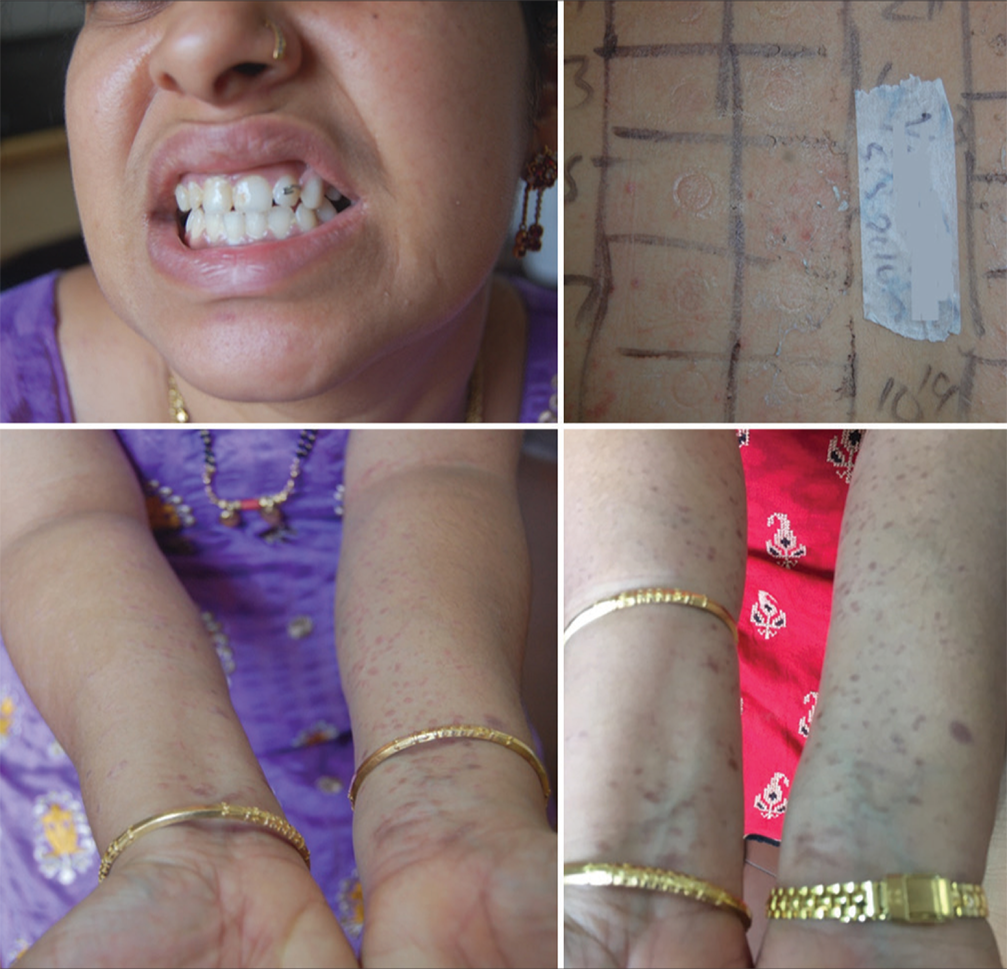
- Dental prosthesis with lichen planus showing positive test for nickel and improvement 6 weeks after avoiding nickel.
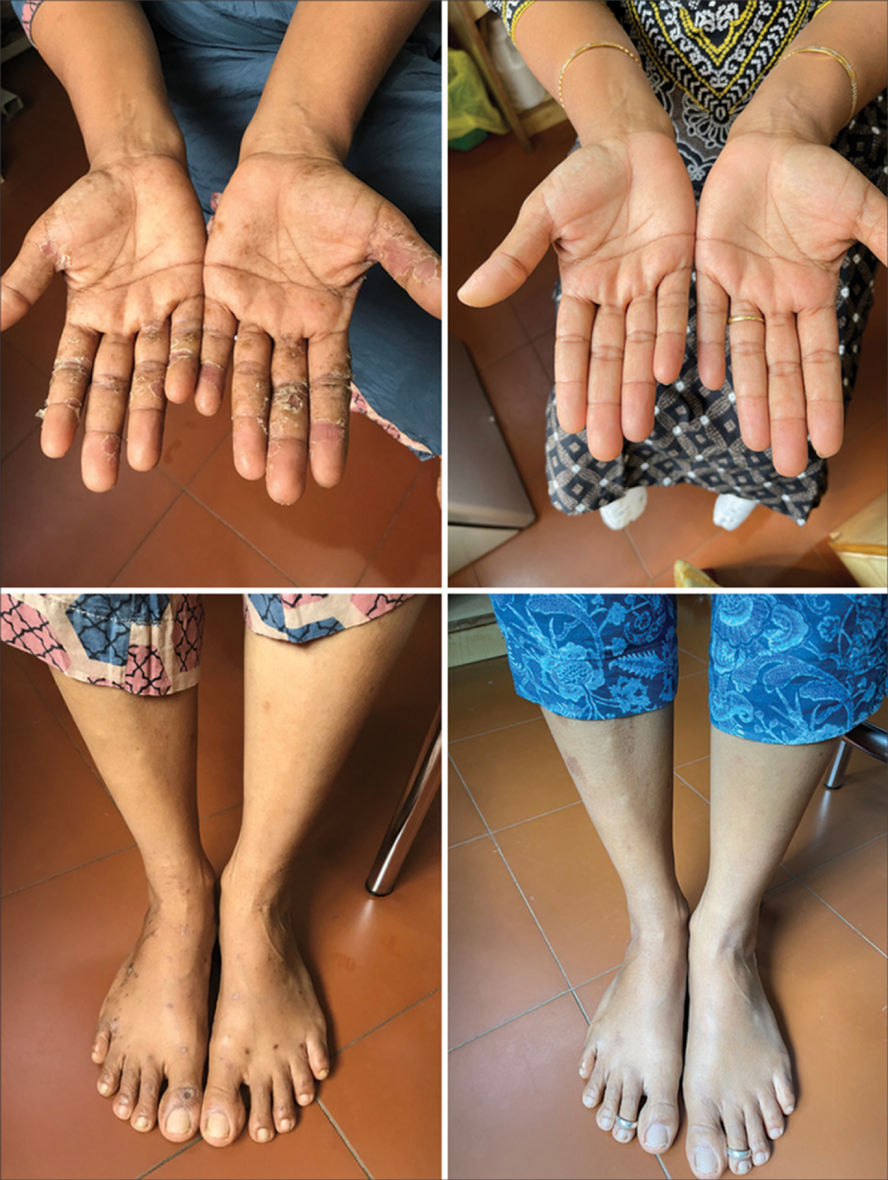
- Nickel-positive lichenoid eruption and pompholyx showing improvement after elimination.
- Nickel-positive lichenoid eruption before and after a low nickel diet.
Of the 37 cases that showed positive tests for parthenium, 27 were females, and 10 were males, of age 5 –70 years. Thirteen were positive for parthenium alone, and the remaining 24 had positive tests for parthenium along with other allergens. Among the parthenium-positive cases, 6 cases had PAD, five had NE, five had FM with LPP or PAD, four had LE, 3 had LP, 3 had AE, and the remaining 11 cases had ABCD, urticaria, chronic dermatitis of hands/feet, mucosal LP, PLE, and vulvar dermatitis [Figure 8; Table 3]. Parthenium-positive cases were advised to avoid edible plants of the Compositae family, such as lettuce, celery, carrot, endive, chicory, sunflower seeds and oil, watermelon, and herbal preparations containing chamomile, feverfew, bisabolol, and echinacea. All patients showed improvement on dietary elimination of foods belonging to the Compositae family and maintained the remission on avoiding the culprit foods. Some of the parthenium-positive patients had worsening of their conditions following ingestion of herbal medicines.
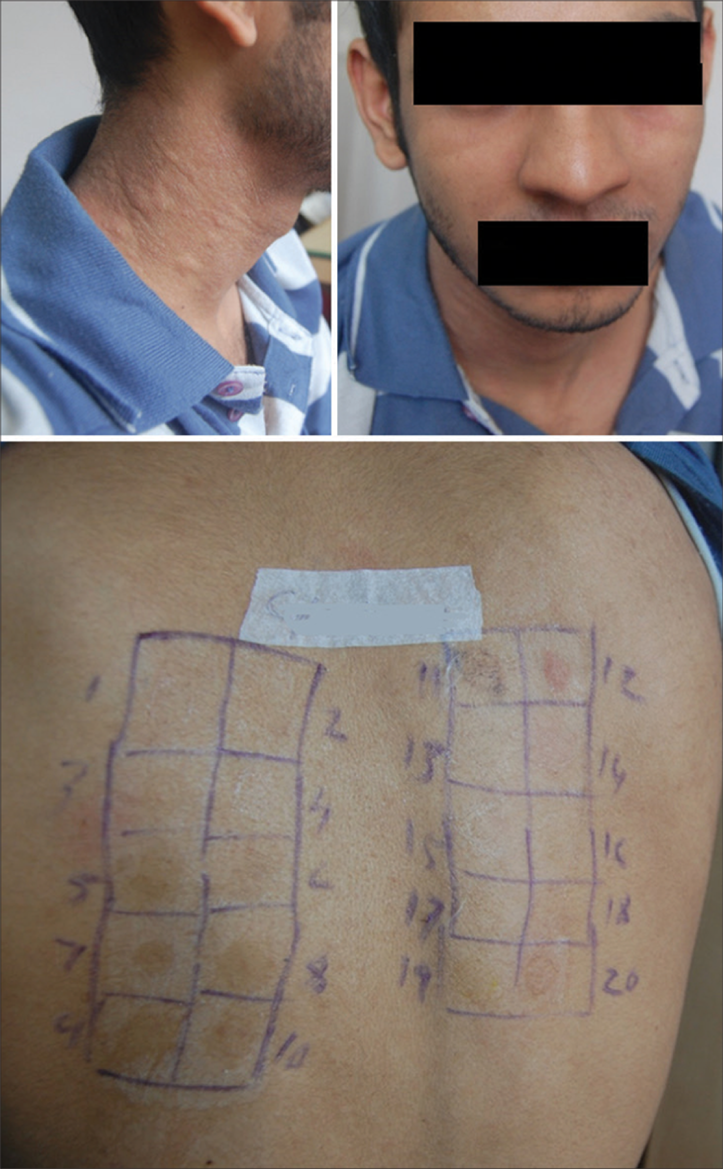
- Airborne contact dermatitis showing positive test for parthenium.
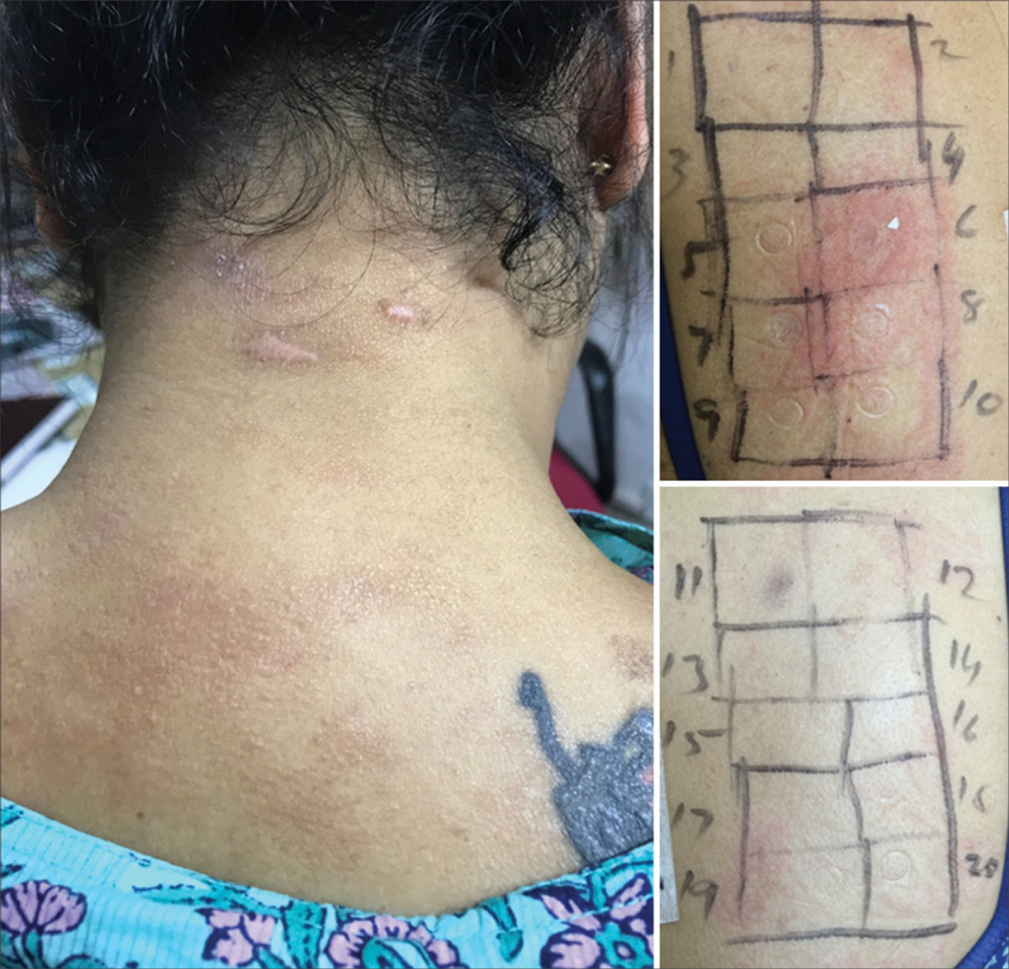
Of the 19 cases positive for fragrance mix, 16 were females, and 3 were males aged 18–65 years. Six were positive for fragrance alone, and 13 were positive for fragrance along with other allergens. Along with FM, LPP, PAD, and PLE, test for fragrance was positive in cases of NE, LE, and LP as overlapping dermatoses [Figures 9 and 10; Table 3]. The fragrance-sensitive patients showed satisfying improvement on avoiding flavored foods and beverages, citrus fruits, cinnamon, clove, vanilla, and herbal concoctions containing aromatic substances.
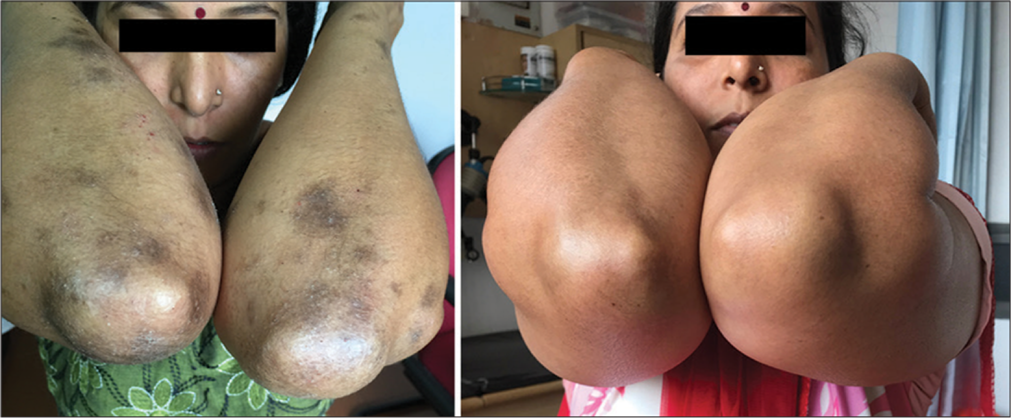
- Pigmented contact dermatitis (facial melanosis) showing positive test for fragrance.
- Facial melanosis and photoaggravated dermatitis showing positive test for paraphenylenediamine (hair dye) and fragrance.
Of the 17 cases positive for PPD, 10 were females and 7 were males, of 14 to 62 years. Three cases tested positive for PPD alone, and 14 cases tested positive for PPD along with other allergens. In addition to FM and PAD, the test for PPD was positive in PLE, chronic dermatitis of hands/feet, LE, NE, and ACD [Table 3]. Patients sensitive to PPD improved on stopping hair dye and maintained improvement so long as they continued the elimination [Figure 11]. Those PPD-positive cases on medications containing sulfonylureas and thiazides and presenting with recurrent dermatitis on the sun-exposed areas improved on changing these medications.
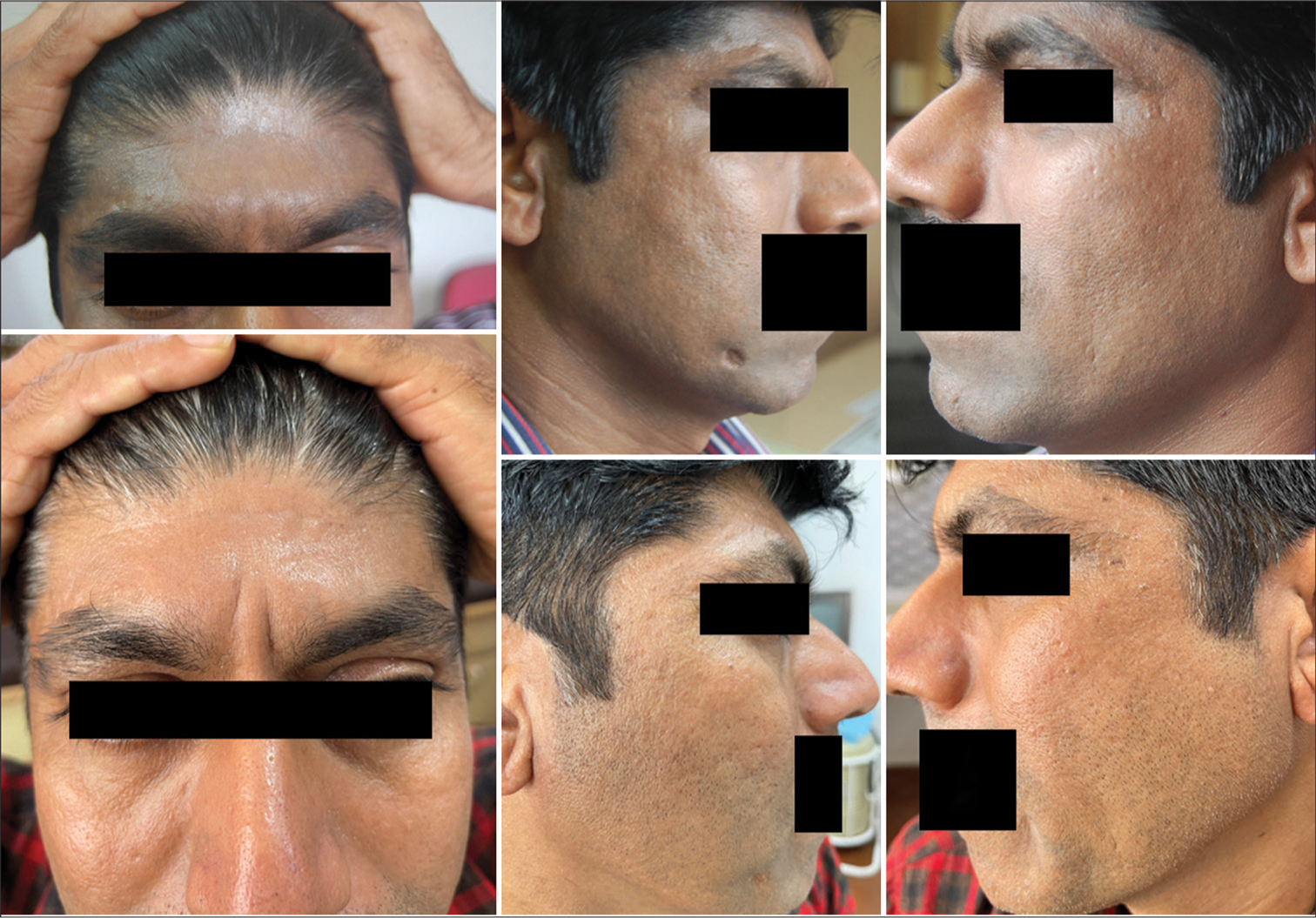
- Facial melanosis positive for paraphenylenediamine and parthenium before and after elimination of allergens.
DISCUSSION
Systemic provocation through ingestion, inhalation, or absorption of allergen/s in sensitized patients can produce many different types of dermatitis and dermatoses.[2,5,6] In the present study, patch tests were found to be positive in non-eczematous conditions such as LP, LE, LPP, and PCD, in addition to those in eczematous conditions. Most of the positive cases had overlapping clinical features and more than one allergen positive.
Sensitization to one allergen is known to facilitate sensitization to another unrelated chemical[7], which probably explains the overlap of clinical presentation and positivity for more than one allergen in the present study.
As observed in other studies, the present study also shows a female preponderance in patch test positive cases.[8] This is due to the fact that women come in contact with these sensitizers more often through household work, leading to a breach in the skin, causing sensitization, and also through the use of cosmetics. Female preponderance in nickel and fragrance sensitivity in the present study could also be attributed to these factors.
In the present study, nickel was the most common allergen, positive in 40 (32%) cases, as also reported in other studies.[9] The most common presentation of nickel allergy in the present study was FM/LPP, as also reported by Tienthavorn et al.[10] Systemic reactions due to dietary ingestion of nickel, such as generalized eczematous reactions, dyshidrotic hand eczema (pompholyx) and pseudo atopic dermatitis, LE, LP, PCD, and perianal pruritus, have been reported, as seen in the present study.[11]
Industrialization, food processing, and modern living have increased cutaneous exposure to metals and have contributed to the increase in cases of metal allergy, particularly nickel and cobalt, which are strong skin sensitizers.[11] Epidemiological studies have revealed that a high percentage of patients with metal allergies also had atopic dermatitis, allergic rhinitis, and food allergies.[12] Yunizar et al. reported higher infiltration of T cells and thymic stromal lymphopoietin (TSLP) receptor signaling and tumor necrosis factor-alpha (TNF-a) production in the oral LP lesions of the metal allergy-positive patients than in the metal allergy-negative patients.[13] Therefore, metal allergies through promoting TNF-a production may increase susceptibility to chronic dermatitis and dermatoses. This may explain metal allergy being positive in varied skin conditions and their subsequent improvement on avoidance in the present study, underlining the fact that metals, particularly nickel, are important causes for SCD mimicking many skin conditions.
Parthenium hysterophorus, belonging to the Compositae family, is the most common plant allergen in India.[14] In sensitized individuals, it can cause a spectrum of clinical manifestations, such as airborne pattern, chronic actinic dermatitis-like presentation, mixed pattern dermatitis, exfoliative dermatitis, widespread dermatitis, lichen nitidus, and other rare patterns.[14,15] Contact sensitization to parthenium may predispose individuals to systemic reactions following airborne inhalation of parthenium plant particles and following oral consumption of plants that belong to the Compositae family.[6] Mahajan et al. have reported widespread discrete and confluent erythematous-squamous rash following inhalation of fresh parthenium plant particles.[6] Oral swelling, perianal pruritus, and dermatitis of the trunk and arms have also been reported in a sensitized person following consumption of Chamomile tea.[16] In a study conducted by Tienthavorn et al., in addition to PCD, patients with a provisional diagnosis of ashy dermatoses and LPP had positive patch tests in 40 and 36.5% cases, respectively, the most common allergen being nickel, followed by fragrance mix, cobalt, and hair dye.[10] Dandle et al. documented a positive reaction to PPD in 8.6% of cases with FM.[17] Fragrance from personal care products is well documented as a cause of FM. Nickel sensitization as a cause of FM also needs to be considered. Both nickel and cobalt can be common skin sensitizers, not only through their existence in household alloys, but also in different consumer products such as colored cosmetics that contain mineral pigments such as iron and manganese having impurities of nickel and cobalt.[18]
LP due to sensitivity to metals, particularly nickel, has been reported [19]. LPP being a variant of LP, it may not be surprising to find positivity to nickel as a sensitizer in LPP. This finding needs to be supported by further studies, as this is a common occurrence. Moreover, identification and early intervention with a low-nickel diet can help prevent the progression of this condition, as observed in the present study.
CONCLUSION
SCD, or systemic reaction to known sensitizers, should be thought of in patients presenting with recurrent dermatitis and overlap of more than one type of dermatoses. Therefore, indications for patch testing must be widened to unravel and confirm the unsuspected sensitizers in varied dermatological conditions. Dietary elimination of certain sensitizers detected in patch tests showed long-term remission, which could not be achieved with standard treatment alone. Breach in the skin barrier due to the existing conditions may increase the chances of sensitization and such sensitization could lead to systemic contact dermatitis manifesting as chronic, recurring and overlap dermatoses.
Ethical approval
The study was conducted in accordance with Helsinki Declaration as revised in 2013 and was approved by the Institutional Ethics Committee (No.: Ethics/2014-15/PBSSCD, dated April 7, 2014).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Noneczematous contact dermatitis. ISRN Allergy. 2013;2013:361746.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic contact dermatitis: Current challenges and emerging treatments. Curr Treat Options Allergy. 2014;1:348-57.
- [CrossRef] [Google Scholar]
- Diagnostic approach in allergic and irritant contact dermatitis. Expert Rev Clin Immunol. 2010;6:291-310.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing In: Rycroft RJ, Menné T, Frosch PJ, Lepoittevin JP, eds. Textbook of contact dermatitis. Berlin, Heidelberg: Springer; 2001.
- [CrossRef] [Google Scholar]
- Systemic contact dermatitis. Indian J Dermatol Venereol Leprol. 2006;72:99-102.
- [CrossRef] [PubMed] [Google Scholar]
- Parthenium dermatitis: Is it a systemic contact dermatitis or an airborne contact dermatitis? Contact Dermatitis. 2004;51:231-4.
- [CrossRef] [PubMed] [Google Scholar]
- Management of contact dermatitis due to nickel allergy: An update. Clin Cosm Investig Dermatol. 2009;2:39-48.
- [CrossRef] [PubMed] [Google Scholar]
- Standard patch test results and clinical relevance: A cross-sectional study of 10-year retrospective experience. Indian J Dermatol. 2022;67:258-64.
- [CrossRef] [PubMed] [Google Scholar]
- The European Surveillance System of Contact Allergies (ESSCA): Results of patch testing the standard series, 2004. J Eur Acad Dermatol Venereol. 2008;22:174-81.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing and Histopathology in Thai patients with hyperpigmentation due to erythema dyschromicum perstans, lichen planus pigmentosus, and pigmented contact dermatitis. Asian Pac J Allergy Immunol. 2014;32:185-92.
- [CrossRef] [PubMed] [Google Scholar]
- Metal allergy and systemic contact dermatitis: An overview. Dermatol Res Pract. 2012;2012:749561.
- [CrossRef] [PubMed] [Google Scholar]
- A multi-institutional survey of clinical symptoms and prosthodontic treatment for metal allergy to dental materials. J Jpn Assoc Dent Sci. 2015;34:44-8.
- [Google Scholar]
- Metal allergy mediates the development of oral lichen planus via TSLP-TSLPR signaling. J Clin Med. 2022;11:519.
- [CrossRef] [PubMed] [Google Scholar]
- Parthenium dermatitis in India: Past, present and future. Indian J Dermatol Venereol Leprol. 2012;78:560-8.
- [CrossRef] [PubMed] [Google Scholar]
- Changing clinical patterns of parthenium dermatitis. Contact Dermatitis. 1997;37:128.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-3.
- [CrossRef] [PubMed] [Google Scholar]
- Patch test in facial melanosis. Poster presentation. P97, 21st International Pigment Cell Conference 20-24 September 2011. Pigment Cell Melanoma Res. 2011;24:742-863.
- [CrossRef] [Google Scholar]
- Presence of impurities of nickel and cobalt in facial cosmetic pigments and their dissolution into artificial sweat. Contact Dermatitis. 2022;87:550-3.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-7.
- [CrossRef] [PubMed] [Google Scholar]






