Translate this page into:
A novel pitch against the itch
*Corresponding author: Shruthi Pavana Janardhanan, Everything Skin and Hair Medical and Aesthetic Dermatology Clinic, Mumbai, Maharashtra, India. shruthjan@yahoo.com.sg
-
Received: ,
Accepted: ,
How to cite this article: Janardhanan S, Saraogi P. A novel pitch against the itch. Indian J Skin Allergy. 2024;3:122-4. doi: 10.25259/IJSA_13_2024
Abstract
About 25% of patients suffering from chronic spontaneous urticaria (CSU) are refractory to first-line therapy, making treatment challenging in these cases. There are limited options in the treatment ladder for urticaria as omalizumab is more effective in patients with raised immunoglobulin E levels; also, cost and feasibility of administration are limiting factors. Dose-related side effects often limit cyclosporine’s greater efficacy. We started tofacitinib for our 68-year-old patient who had been suffering from refractory CSU for the past 12 years and observed quick control of signs and symptoms starting within 2 weeks and good long-term control for over 9 months of which last 6 months were monotherapy.
Keywords
Chronic spontaneous urticaria
Refractory
Cyclosporine
Tofacitinib
INTRODUCTION
Chronic spontaneous urticaria (CSU) is urticaria/angioedema or both for 6 weeks or more with no known inducible factors. It is considered refractory when it stops responding to first-line and subsequent recommended therapies. The overall goal of treatment is to help patients be free of signs and symptoms until spontaneous remission, necessitating continuous pharmacological therapy.[1]
CASE REPORT
A 68-year-old female presented with a 12-year history of recurring CSU with partial response to treatments and dose-limiting side effects. A step-up approach according to the latest International EAACI/GA2LEN/EuroGuiDerm/APAAACI guidelines, including updosing of second-generation H1 antihistamines 4 times, adding H2 antihistamines in various combinations had been followed for the past 3 years. She was not open to omalizumab due to the cost and feasibility of administration. Further, investigations revealed low immunoglobulin (Ig) E levels (22 IU/ml) making her a poor candidate for omalizumab.[2] She was treated 5 years ago with cyclosporin A (CysA) with very good results, and the same was restarted at 1 mg/kg and increased to 3 mg/kg with a satisfactory reduction of urticaria activity score (UAS7). Unfortunately, due to dose-related hypertension, CysA had to be tapered down and maintained at a low dose of 50 mg/day. This led to frequent exacerbations [Figure 1], with five episodes of angioedema in 4 months, warranting short courses of low-dose oral steroids. Her UAS7 remained high at each review, with scores consistently above 35. Tofacitinib was started at 5 mg twice daily after relevant investigations, and within 2 weeks, her UAS7 score significantly improved to <7. CysA was then tapered and discontinued completely by week 4. She maintained well with lower doses of oral antihistamines (Bilastine 20 mg OD and Fexofenadine 180 mg OD). After 2 months, she discontinued antihistamines on her own and was on monotherapy of tofacitinib 5 mg BD. It is 9 months since the start of therapy and 6 months of monotherapy of tofacitinib 5 mg BD with regular blood pressure monitoring; her UAS7 is <7 with no therapy-related side effects [Figure 2].
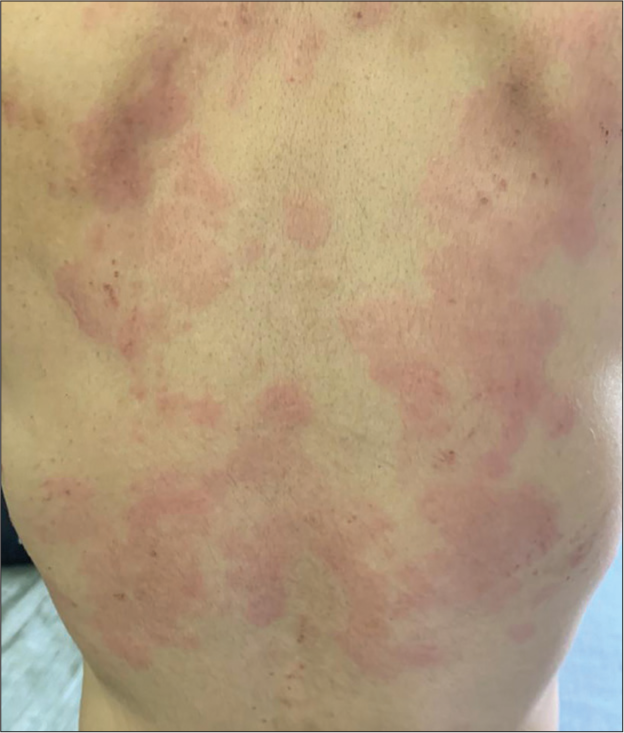
- Urticarial lesions over the back during acute flare up.
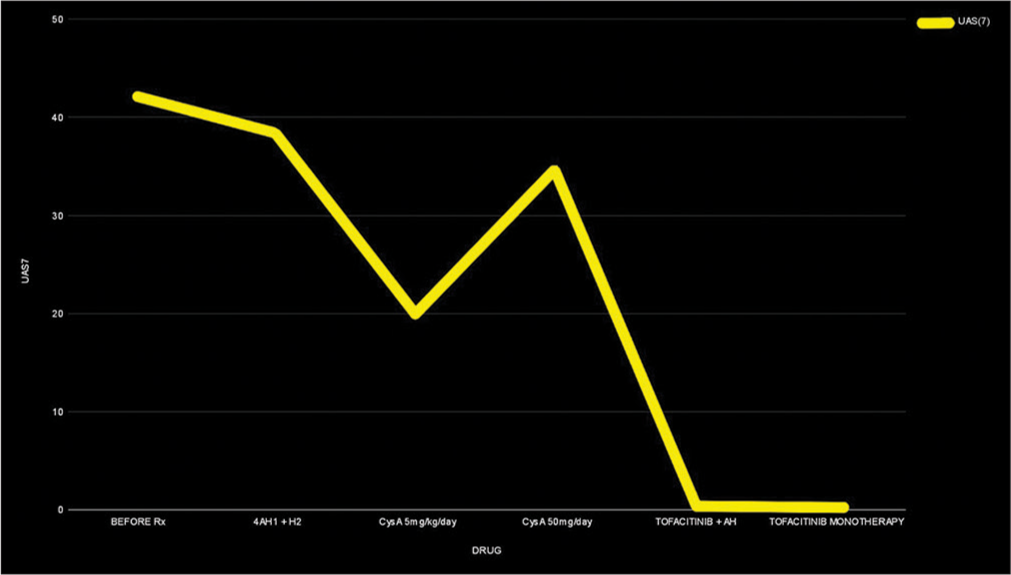
- Graph showing the UAS7 scored recorde corresponding to the treatment given.
DISCUSSION
CSU negatively impacts the quality of life, often complicated by anxiety and depression in over 30% of patients.[3] The pathogenesis involves an autoimmune pathway characterized by the presence of IgE autoantibodies (type I or autoallergic) or IgG autoantibodies (type IIb).[4] The common pathway involves mast cell activation, leading to vasodilation, inflammation, and sensory neural activation.[4]
Over 25% of patients do not show satisfactory results with first-line treatment of four-fold dose escalation of second-generation H1 antihistamines.[5] Omalizumab is a Food and Drug Administration-approved recombinant humanized monoclonal antibody against IgE for the treatment of antihistamine-refractory CSU. It binds to free IgE and inhibits FceRI receptor interaction on basophils and mast cells, preventing their activation.[6] Those with type IIb CSU (low IgE levels), like our patient, are less likely to respond to omalizumab and often have delayed onset of response.[4]
CysA is an off-label add-on therapy in patients with severe CSU who are refractory to first and second line therapies.[7] CysA binds to cyclophilin, therefore inhibiting calcineurin and reducing the production of inflammatory cytokines involved in IgE-related mast cell activation. The results of a meta-analysis conducted by Kulthanan et al. show that cyclosporin in low doses (<4 mg/kg) for 12 weeks significantly improves clinical severity in 70% of patients.[8] The role of CysA is best described as a short-term crisis buster and is not preferred for long-term control.
Other drugs such as azathioprine, dapsone, mycophenolate mofetil, tumor necrosis factor-alpha inhibitors, intravenous immunoglobulin, and even dupilumab have been used in urticaria with limited studies as an off-label use.[6] Newer drugs such as ligelizumab (anti-IgE) and Bruton kinase inhibitors are also being evaluated in Phase 3 and Phase 2 trials, respectively.[6]
Tofacitinib citrate is a Janus kinase 1/3 (JAK1/3) inhibitor that falls under the category of small molecules with short half-lives that block intracellular signaling of multiple key cytokines associated with urticaria and, thus, can provide steroid sparing advantage with anti-inflammatory and immunomodulatory effects. A recent review by Darougar et al. mentions that the increased expressions of IL-9, IL-10, and JAK/STAT3 in CSU lead to downstream inflammatory cytokines such as IL-4, IL-13, and IL-31 that can be blocked by JAK/STAT inhibitors.[9] There are limited studies/reports on the efficacy of JAK inhibitors in cases of CSU. Mansouri et al., in 2022, presented a case series of five patients with urticaria and urticarial vasculitis successfully treated with oral tofacitinib.[10] A case report by Fukunaga et al. showed the efficacy of ruxolitinib (JAK1/3 inhibitor) in chronic refractory urticaria when added for complicated primary myelofibrosis.[11] Li et al. documented the successful treatment of Schnitzler syndrome, characterized by recurrent chronic urticaria with fever, fatigue, rapid weight loss, and poor response to antihistamine treatment with a combination of oral tofacitinib and colchicine.[12]
Oral JAK inhibitors have been FDA-approved in Dermatology only since 2022 for alopecia areata, psoriasis, and atopic dermatitis,[13] and safety studies in these diseases have displayed that the most common adverse effects observed in ≥ 5% of patients included upper respiratory tract infection, nasopharyngitis, nausea, headache, and acne.[14] The review by Samuel et al. also reported low rates similar to placebo of venous thromboembolism, major adverse cardiovascular events, and malignancy in their use in clinical trials in dermatology.[14]
Although tofacitinib is a newcomer in the field of urticaria, it is backed by safety data available from its use in the field of rheumatology, where its intended use is continuous long-term with monitoring. The analytical study by a rheumatoid arthritis (RA) clinical group spanning 9.5 years of cumulative tofacitinib exposure in >7000 patients concluded safety profile of tofacitinib is similar to Disease-Modifying Antirheumatic Drugs (DMARDs) with the exception of a slightly higher incidence of Herpes Zoster.[15] In 2020, the FDA even approved the use of tofacitinib in children 2 years and older with active polyarticular juvenile idiopathic arthritis.[16] Curtis et al. extensively evaluated the safety and efficacy of tofacitinib in patients with moderate to severe RA in older age groups (≥65 years) and found that though the efficacy in treatment is similar to that of younger age groups, there is a numerically higher risk of serious infectious events in older patients.[17] Therefore, it is important to comprehensively evaluate the patients’ baseline risk factors for complications and comorbid conditions to assess the benefit of JAK inhibitors.
As with other medications, the tapering of tofacitinib is a definite challenge as the CSU disease itself is active in phases and may wax and wane. Step-down protocols for CSU that can be used are either well-regulated tapering strategies over a longer period or abrupt stoppage of treatment after 6 months to 1 year of complete response, and often, this choice has no relation to the risk of relapse.[18] It is important to counsel patients that the risk of relapse remains high with any treatment discontinuation as none of the drugs is disease-modifying, including omalizumab, and they may need multiple re-treatment cycles.[18] The tapering of tofacitinib in CSU may be tried as in a case report of alopecia areata where 5mg BD was given over 8 months for complete response, then reduced to 5mg OD for the next 5 months, followed by 5mg twice a week, and this maintenance therapy maybe needed to continue the disease remission.[19] Further studies would be required to decide the tapering regimen of JAK inhibitors in CSU.
CONCLUSION
The relative safety and minimal contraindications for tofacitinib combined with lower cost, considering the availability of generic medication in India, make it an ideal stepladder in the treatment of CSU. In comparison to conventional modalities, the efficacy of tofacitinib offers a major breakthrough with long-term use safety data. After a full literature search, to our knowledge, this is the first report on the use of tofacitinib monotherapy for CSU in the Indian scenario. We believe controlled studies in large populations using this molecule will further the road to the establishment of tofacitinib and other JAK/STAT molecules in dermatologists’ toolboxes for urticaria.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734-66.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of treatment response in chronic spontaneous urticaria. Allergy. 2021;76:2965-81.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of urticaria in Indian settings: Skin allergy research society's guideline-2022. Indian J Dermatol. 2022;67:73243.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol. 2017;139:1772-81.e1.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226-36.
- [CrossRef] [PubMed] [Google Scholar]
- Current and emerging therapies for chronic spontaneous urticaria: A narrative review. Dermatol Ther (Heidelb). 2023;13:1647-60.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic urticaria: Advances in understanding of the disease and clinical management. Clin Rev Allergy Immunol. 2021;61:424-48.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclosporine for chronic spontaneous urticaria: A meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-99.
- [CrossRef] [PubMed] [Google Scholar]
- Janus-kinase Inhibitors in pathogenesis and management of chronic urticaria: A review of the literature. J Pediatr Rev. 2023;11:153-62.
- [CrossRef] [Google Scholar]
- Efficacy of oral tofacitinib in refractory chronic spontaneous urticaria and urticarial vasculitis. Dermatol Ther. 2022;35:e15932.
- [CrossRef] [Google Scholar]
- Efficacy of oral ruxolit inib in a patient with refractory chronic spontaneous urticaria. Acta Derm Venereol. 2018;98:904-5.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: Successful treatment with tofacitinib and colchicine in a patient with Schnitzler syndrome. Int J Rheum Dis. 2023;26:160-3.
- [CrossRef] [PubMed] [Google Scholar]
- Janus-kinase inhibitors in dermatology: A review of their use in psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versushost disease. Indian J Dermatol Venereol Leprol. 2024;90:30-40.
- [CrossRef] [PubMed] [Google Scholar]
- A review on the safety of using JAK inhibitors in dermatology: Clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13:729-49.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety of tofacitinib up to 9.5 years: A comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395.
- [CrossRef] [PubMed] [Google Scholar]
- FDA approvals in 2020 represent many firsts for children. 2020. Available from: https://www.fda.gov/media/145481/download?attachment [Last accessed on 2024 Mar 07]
- [Google Scholar]
- Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin Exp Rheumatol. 2017;35:390-400.
- [Google Scholar]
- Stepping down treatment in chronic spontaneous urticaria: What we know and what we don't know. Am J Clin Dermatol. 2023;24:397-404.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of oral tofacitinib dose tapering in a case of alopecia areata universalis. Skin Appendage Disord. 2021;7:36-40.
- [CrossRef] [PubMed] [Google Scholar]






