Translate this page into:
Chronic actinic dermatitis
*Corresponding author: Shanmuga Sekar C, Department of Dermatology, PSG Institiute of Medical Sciences and Research, Coimbatore, Tamilnadu, India. drshanmugasekar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sekar SC. Chronic actinic dermatitis. Indian J Skin Allergy. 2024;3:41-4. doi: 10.25259/IJSA_10_2024
Abstract
Chronic actinic dermatitis (CAD) is a chronic immune-mediated photo-dermatosis causing pruritic eczematous lesions, infiltrated lichenified papules,and plaques on sunexposed skin. It commonly affects elderly males who are chronically exposed to sunlight. The photosensitivity is mostly to ultraviolet (UV) B wavelengths and less frequently to ultraviolet (UV)A and visible light. In this article, the pathogenesis, clinical features, investigations, and treatment of CAD will be discussed.
Keywords
Dermatitis
Ultraviolet radiation
differentiation (CD) 8 cells
INTRODUCTION
Chronic actinic dermatitis (CAD) is a chronic immune-mediated photo-dermatosis which presents as pruritic eczematous lesions, infiltrated lichenified papules, and plaques over sun- exposed skin. The other synonyms for CAD include photosensitive dermatitis, actinic reticuloid, and persistent light reaction.[1] It commonly affects elderly males of 50–60 years with any skin type. The spectrum involved in CAD is predominantly ultraviolet B (UVB) wavelengths and less commonly to ultraviolet A (UVA) and visible light.[1,2] In this article, the immunopathogenesis, clinical manifestations, and management of CAD will be discussed.
PATHOGENESIS
CAD is a delayed type of hypersensitivity to endogenous photo-induced antigen. These allergens develop due to ultraviolet radiation (UVR) altered molecular changes in DNA.[2] In a normal healthy skin, UV radiation causes immunosuppression by increasing the immunosuppressive cytokines (interleukin [IL]-10, IL-4, and tumor necrosis factor [TNF]-alpha), along with an increase in infiltration of neutrophils, mast cells, and T-regulatory cells (Treg).[3] On exposure to cutaneous antigens, there is production of both effector and regulatory T-cells. After UVR exposure, the generation of Treg cells is not affected but the number of effector T-cells is reduced. The relative increase in Treg cells induces immunosuppression. A second group of cells known as natural killer t (NKT) cells exhibit both natural killer cell and T-cell activity. These NKT cells produce IL-4 (Th2 cytokine),which suppresses the immunity after ultraviolet (UV) exposure. Recent animal model studies have proved that epidermal Langerhans cells are required for generation of Treg cells after an UV exposure. UVR has both direct and indirect effects on cutaneous dendritic cells resulting in immunosuppression [Figure 1].[4]
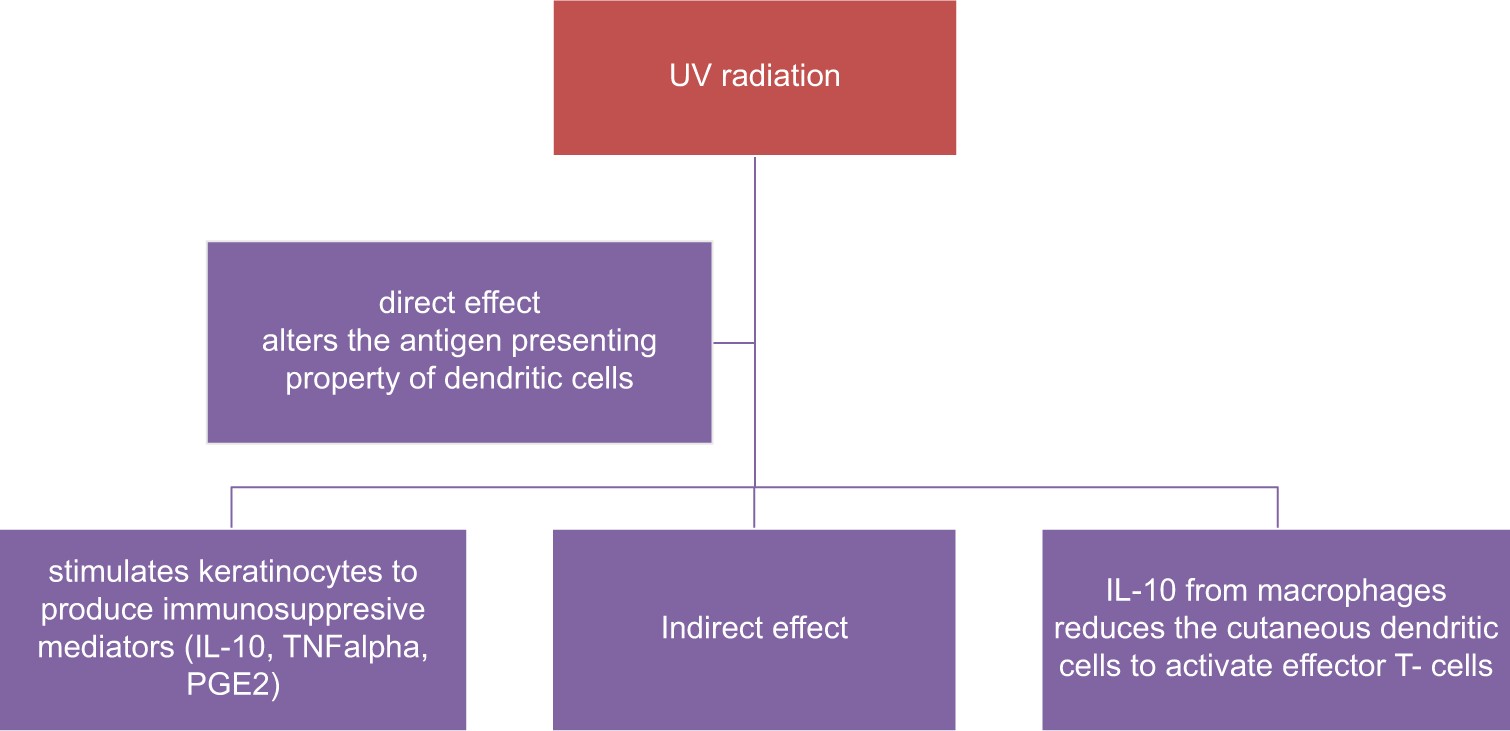
- UV induced immuno-suppression. IL: interleukin, TNFalpha: tumor necrosis factor, PGE2: Prostaglandin E2.
In skin lesions of CAD, there is decrease in the production of these immunosuppressive cytokines along with the decrease in infiltration of neutrophils and mast cells. Biopsy from CAD skin lesions shows leukocyte epidermotropism, dermal infiltrate with predominant T-cells, and keratinocytes expressing major histocompatibility complex-II which are features of delayed-type hypersensitivity reaction. There are two theories involved in pathogenesis of CAD. The first theory postulates that in CAD patients, there is an abnormal exaggerated immune response to photo-antigen. The second theory explains the cross-reactivity between an endogenous and photo-allergen. This, further, explains the persistence of immune response to endogenous antigen even after the removal of the photoallergen.[3,4] In skin lesions of CAD, there is an increase in infiltration of CD4+ cells, CD8+ cells, IL-17 producing Th17 cells, and interferon-gamma (IFN-γ) producing natural killer cells which activate the inflammatory cascade. There is also depletion of Treg cells predisposing to an autoimmunity-like condition. The recall reaction induced by a photo-contact allergen in CAD is due to an increase in the number of CD69+ resident T-memory cells. Medical interventions for CAD involve targeting these immune molecules involved such as interferon-gamma (IFN-γ), IL-17, and CD4/CD8 cells. Animal model studies have demonstrated that both UVA and UVB exposure resulted in increased expression of IL-36γ (IL-1 family cytokines) which, further, activates the NF-kB pathway and increases inflammation. The other cytokines which are increased in CAD include IL-8, CCL-17, and CCL-18.[3,5]
Pyroptosis is an inflammatory programmed cell death pathway characterized by swelling of cells, pore formation on plasma membranes, and elevated inflammatory cytokines. The important pyroptosis-mediated proteins are caspase 1, 4 and gasdermins. N6-methyladenosine (m6A) is an important ribonucleic acid (RNA) modification for messenger RNA and non-coding RNA which is crucial in regulating pyroptosis. In CAD, UVB radiation increases cell apoptosis, release of inflammatory cytokines (IL-1β and IL-18), and pyroptosis by increasing the expression of ALKBH5 (m6A demethylase AlkB homolog 5).[6]
CLINICAL FEATURES
It is a chronic condition with recurrent episodes affecting the quality of a patient’s life.[1] The skin of CAD resembles chronic eczema. It presents as extremely pruritic, red to hyperpigmented, dry and thickened lesions predominantly on the face, neck, chest, back of hands, and forearms with sparing of skin folds, skin creases, and upper eyelids [Figures 2 and 3]. CAD can also affect the covered areas of the skin. The lesions are exacerbated during the summer season. Infiltration of skin can accentuate the facial skin markings resembling leonine facies. Hairs of eyebrows and eyelashes can become short or lost due to persistent itching. Severe cases of CAD can present as erythroderma. In India, patients with CAD can present with contact sensitivity to certain allergens such as parthenium hysterophorus (Compositae family), rubber, metals, and perfumes. The eczematous skin in CAD enables easier penetration of airborne allergens causing contact dermatitis. The oleoresins from compositae plants may induce the photo-aggravation of CAD.[7] The diagnostic criteria for CAD include (1) chronic dermatitis with pruritus and lichenified papules over sun-exposed areas, (2) reduced minimal erythema dose (MED) values to UVL radiation or visible light, and (3) histopathology features of chronic dermatitis, with or without the presence of lymphoma-like changes.[8]
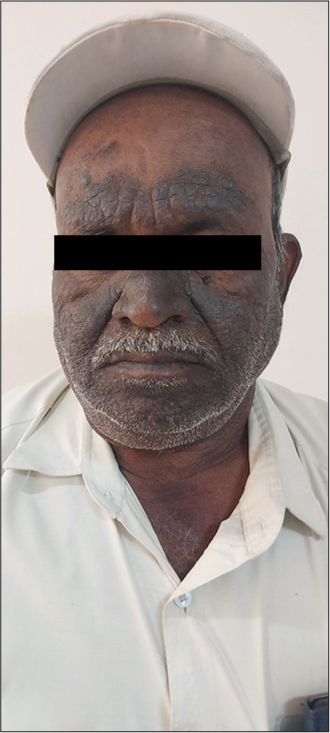
- Hyperpigmented, lichenified plaques involving the face with relative sparing of the eyelids and folds. Image courtesey Prof Sathish B Pai. Professor Department of Dermatology, Venereology and Leprosy, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India
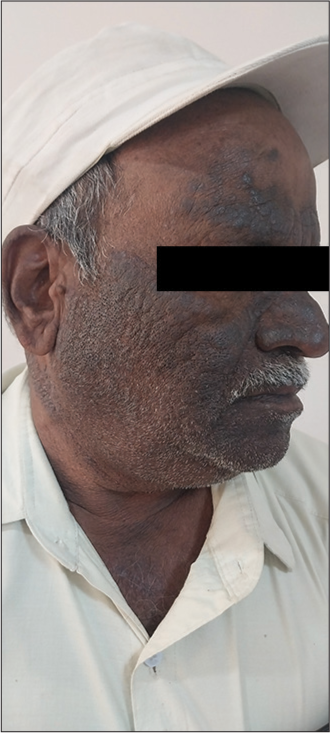
- Involvement of the ear lobe in a patient with chronic actinic dermatitis. Image courtesey Prof Sathish B Pai. Professor Department of Dermatology, Venereology and Leprosy, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India
Histopathology of CAD includes spongiosis, acanthosis, exocytosis, perivascular lymphocytic infiltrate with eosinophils, and increased dermal dendrocytes. There is an increase in elastic fiber degeneration and increased T-lymphocyte infiltration which resembles cutaneous T-cell lymphoma (CTCL). The dendrocytes in CAD are more prominent and are often multinucleated which differs from CTCL. High CD4:CD8 ratio is seen in CTCL, but this ratio is reversed in CAD due to an increase in the infiltration of CD8 cells. There is the involvement of mast cells in the early stage with the involvement of dendritic cells and tissue-resident memory T-cells at the chronic stage of disease.[8] T-cell receptor gene arrangement analysis and immunohistochemistry will be helpful in further differentiating CAD from CTCL.
Dermoscopic findings are non-specific and it may demonstrate the presence of an erythematous background with telangiectasia.
PHOTO TESTING
Photo-testing is performed to evaluate immunologically mediated photo-dermatoses such as CAD, solar urticaria, and polymorphous light eruption. The principle behind photo-testing is to determine the UV spectrum causing photo-dermatoses and plan the management accordingly. Patients are instructed to stop the use of topical steroids or any immunosuppressants two weeks prior to the procedure and to stop antihistamines 72 h before the procedure. A template with several windows of size (2 cm × 2 cm) is placed on normal skin with preferred site over back or abdomen. The skin is irradiated with incremental doses of UV and visible light. The common light source used for photo-testing is a high-powered (xenon and metal halide) lamp with filters along with hand-held light guides. The reading is taken 24 h post-irradiation for determining the MED. The MED is the dose of UVR that produces a perceptible erythema covering the entire irradiated area. In patients of CAD, the values of MEDs to UVB and/or UVA and/or visible light exposure may be reduced.[9] A study on clinical correlation of photo-patch test is mentioned in Table 1.[10]
| Patch testing (101 patients) and photo-patch testing (86 patients) in chronic actinic dermatitis | Positive results in patch (70%) and photopatch test (12.8%) for parthenium, perfume mix and balsam of Peru |
| Somani (a study on chronic actinic dermatitis among nine patients)[10] | Positive results in photopatch test (77.8%) for parthenium, fragrance, colophony, rubber, and sunscreens |
DIFFERENTIAL DIAGNOSES
The clinical manifestations of CAD closely resemble air-borne contact dermatitis (ABCD), photo-aggravated allergic contact dermatitis, atopic eczema, or photosensitivity secondary to drugs. Both CAD and allergic contact dermatitis show increased expression of intercellular adhesion molecule-1. The differential diagnoses are listed in Table 2. Detailed clinical history and sites of skin lesions help to differentiate between these disorders [Figure 4]. Drug-induced photosensitivity will have a history of drug intake prior to the onset of lesions. Family history of atopy and increased serum IgE will point more toward atopic dermatitis. CAD usually spares the skin creases, upper eyelids, area beneath the ear lobe, and lower lips but ABCD involves the entire face including the skin creases.[10]
| Airborne contact dermatitis |
| Atopic eczema |
| Drug photosensitivity |
| Photo-aggravated contact allergy |
| Subacute cutaneous lupus erythematosus |
| Cutaneous T-cell lymphoma |
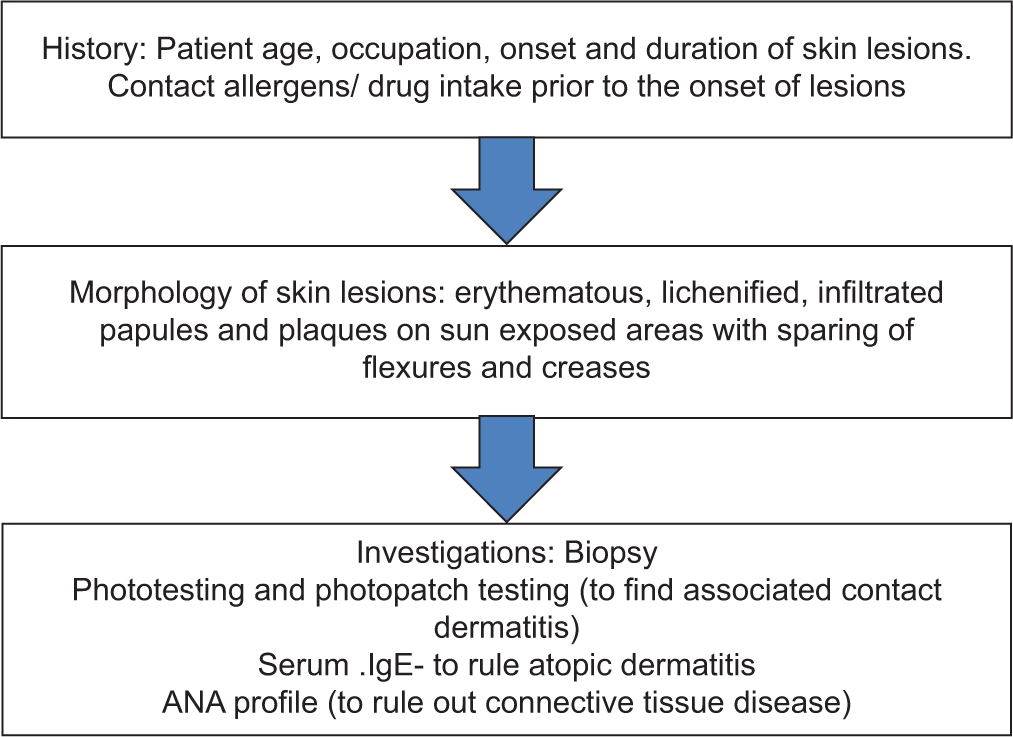
- Flow chart: Clinical approach to chronic actinic dermatitis. IgE: Immunoglobulin E, ANA: Antinuclear antibody
MANAGEMENT
Management of CAD includes strict counseling of the patients about photoprotective measures and the use of broad-spectrum sunscreens with high sun protection factor (SPF). The topical agents for treating CAD include corticosteroids, emollients, and calcineurin inhibitors.[1] For refractory cases, systemic treatment includes the use of oral corticosteroids, immunosuppressive drugs (ciclosporin, azathioprine, and mycophenolate mofetil), and Psoralen-UVA phototherapy.[11] Azathioprine suppresses the denovo purine synthesis and inhibits the proliferation of T-lymphocytes. It also affects the antigen presenting capacity of the epidermal Langerhans cells, hence reducing the type 4 hypersensitivity involved in CAD.[12] Cyclosporine is an immunosuppressant belonging to the group of calcineurin inhibitors. After forming a complex with cytoplasmic cyclophilin, it inactivates calcineurin phosphorylase, and inhibits the production of NFAT dependent cytokine like IL-2 (nuclear factor of activated T-cells), thereby inhibiting the activation of T-cell pathway.[13] The other alternative drug tried in CAD is thalidomide. It possesses an anti-inflammatory action by inhibiting the synthesis of TNF -alpha, thereby reducing the further T-cell response.[14]
In terms of immunopathogenesis of CAD, medical interventions targeting the immunomodulatory molecules involved such as FoxP3, INF-γ, IL-17, CD4, common-γ chain receptor, CD69, CD4/CD8, IL-17/IFN-γ, and IL-17/FoxP3 can be used in treating CAD patients.[3]
The role of low-dose psoralen + UVA in the treatment of CAD includes a hardening effect, increasing the skin pigmentation and thickening of the stratum corneum. It also provides a protective effect by exerting UVR-induced immunomodulation on human skin.[15] 4-aminoquinoline derivatives such as hydroxychloroquine have antiproliferative, anti-inflammatory, photoprotective, and immunomodulatory effects. It inhibits UV-induced reactions and plays an important role in sun protection.[2]
The novel treatment modalities include the use of dupilumab and upadacitinib. Upadacitinib specifically inhibits JAK1 signaling, halts the activation of T lymphocytes, and thereby inhibits the production of proinflammatory cytokines that drive the disease process.[11] Dupilumab is a human monoclonal antibody that binds to the IL-4 receptor and inhibits the Th2-mediated cytokines (IL-4, IL-13). Since dupilumab inhibits the IL-4 and IL-13 cytokines and may interact with immunopathogenesis of CAD and control the disease process, it indicates the involvement of Th2-mediated pathway in pathogenesis of CAD.[2]
CONCLUSION
CAD is an immunologically mediated photodermatoses that requires counseling about sun protective measures and the need for long-term immunosuppressants for recurrent episodes. Many clinical trials have been done to evaluate the efficacy of biologicals and targeted therapy for the treatment of CAD. The knowledge of immunopathogenesis helps in exploring newer treatment modalities for CAD which targets specific cytokines and helps in control of the disease.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The author certifies that he has obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.V
Financial support and sponsorship
Nil.
References
- Efficacy of methotrexate versus azathioprine in the treatment of chronic actinic dermatitis: A randomized control trial. J Ayub Med Coll Abbottabad. 2022;34(Suppl 1):S644-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clearance of chronic actinic dermatitis with dupilumab therapy in chinese patients: A case series. Front Med (Lausanne). 2022;9:803692.
- [CrossRef] [PubMed] [Google Scholar]
- A postulated model for photoimmunopathogenesis of chronic actinic dermatitis around adaptive immunity, including Th17 cells, Tregs, TRMs, cytotoxic T cells, and/or common-γ chain receptor+ cells. Photodermatol Photoimmunol Photomed. 2023;39:147-54.
- [CrossRef] [PubMed] [Google Scholar]
- Role of interleukin-36γ induced by ultraviolet radiation in chronic actinic dermatitis. Photodermatol Photoimmunol Photomed. 2023;39:598-606.
- [CrossRef] [PubMed] [Google Scholar]
- Deficiency of N6-methyladenosine demethylase ALKBH5 alleviates ultraviolet B radiation-induced chronic actinic dermatitis via regulating pyroptosis. Inflammation. 2024;47:159-72.
- [CrossRef] [PubMed] [Google Scholar]
- Photodermatoses in India. Indian J Dermatol Venereol Leprol. 2012;78(Suppl 1):S1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic actinic dermatitis/actinic reticuloid: A clinicopathologic and immunohistochemical analysis of 37 cases. Am J Dermatopathol. 2014;36:875-81.
- [CrossRef] [PubMed] [Google Scholar]
- Phototherapy in the evaluation and management of photodermatoses. Dermatol Clin. 2020;38:71-7.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic actinic dermatitis--a study of clinical features. Indian J Dermatol Venereol Leprol. 2005;71:409-13.
- [CrossRef] [PubMed] [Google Scholar]
- JAK-ing up chronic actinic dermatitis with upadacitinib. Clin Exp Dermatol. 2023;49:173-5.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation on azathioprine in the treatment of parthenium dermatitis. Indian J Dermatol Venereol Leprol. 2001;67:309-11.
- [Google Scholar]
- A case report of therapeutically challenging chronic actinic dermatitis. SAGE Open Med Case Rep. 2019;7:2050313X19845235.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraviolet A rush hardening for chronic actinic dermatitis: Pilot treatment outcomes. J Dermatol. 2021;48:385-8.
- [CrossRef] [PubMed] [Google Scholar]






