Translate this page into:
Subacute cutaneous lupus erythematosus in a patient of pancreatic cancer secondary to novel albumin-bound paclitaxel chemotherapy
*Corresponding author: Sumit Sehgal, Department of Dermatology, Ananta Institute of Medical Sciences and Research Centre, Udaipur, Rajasthan, India. summitsehgal934@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sehgal S, Jain M, Agrawal S, Khedkar C. Subacute cutaneous lupus erythematosus in a patient of pancreatic cancer secondary to novel albumin-bound paclitaxel chemotherapy. Indian J Skin Allergy 2023;2:100-2.
Abstract
Paclitaxel is commonly used for the treatment of breast, ovarian, gastrointestinal, and carcinoma of the head and neck. Paclitaxel treatment is often associated with hair loss, myalgia, acral erythema, scleroderma and Stevens–Johnson syndrome, peripheral neuropathy, nausea, vomiting, bone marrow suppression, and rarely cardiac electroconductive pathway abnormalities. However, its association with cutaneous/systemic lupus erythematosus has been sparsely described. In this case report, we are discussing a case of nab-paclitaxel-induced subacute cutaneous lupus erythematosus which has been described only once in the published literature.
Keywords
Taxanes
Cremophor EL
Nab-paclitaxel
Cutaneous lupus erythematosus
INTRODUCTION
Paclitaxel is a commonly administered chemotherapeutic agent, derived from the bark of the Pacific yew tree that exerts its chemotherapeutic effect by preventing microtubule breakdown causing cell death.[1] It is commonly used for the treatment of breast, ovarian, gastrointestinal, and carcinoma of the head and neck. Paclitaxel treatment is often associated with hair loss, myalgia, acral erythema, scleroderma, and Stevens–Johnson syndrome, peripheral neuropathy, nausea, vomiting, bone marrow suppression, and rarely cardiac electroconductive pathway abnormalities.[1,2] Drug-induced subacute cutaneous lupus erythematosus (DISCLE) secondary to paclitaxel is a very rare occurrence. Cutaneous lupus erythematosus induced by paclitaxel was first reported by the Adachi and Horikawa in two patients with breast carcinoma.[3] To the best of our knowledge, only six case reports have described the occurrence of DI-SCLE secondary to either novel albumin-bound (nab) paclitaxel or Cremophor EL containing paclitaxel and none from India.[1-5] We are describing a case of DI-SCLE secondary to nabpaclitaxel (Cremophor-free formulation) in a patient with advanced pancreatic carcinoma and it is the second case report linking nab-paclitaxel to subacute cutaneous lupus erythematosus (SCLE), the first being reported in 2013 by Lamond et al.
CASE REPORT
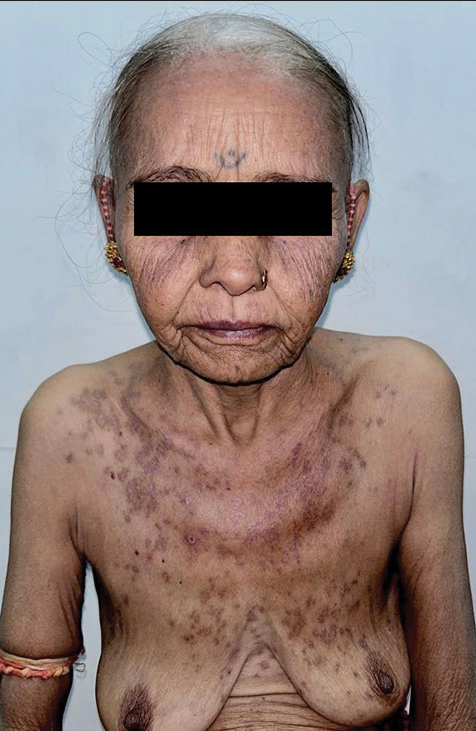
A 59-year-old woman presented with multiple, mildly pruritic scaly papules to a few annular plaques on an erythematous background over the “V” area of the neck and chest for 10–12 days. On closer inspection, erythematous macules with minimal scaling were present bilaterally on the malar eminences [Figure 1]. Mucosae were normal. There was no history of arthralgia, myalgia, and fever or any other constitutional symptoms suggesting the absence of systemic involvement. She also denied itching, burning, or eruption of cutaneous lesions on exposure to sunlight indicating the absence of photosensitive events in the past. Her medical records revealed a history of single nab-paclitaxel infusion for metastatic pancreatic carcinoma 2 weeks back. She could not recall the occurrence of similar-looking lesions in the past. She also reported the absence of any such skin illness in the family. Due to the close association between the intake of a new drug (nab-paclitaxel) and the acute onset of the cutaneous symptoms and their typical morphology, she was thoroughly evaluated for the possibility of the DI-SCLE. Routine investigations were normal except for hypocytic hypochromic anemia and raised ESR (37 mm/h). Histopathological examination suggested the findings of SCLE [Figure 2]. The serum antibody profile revealed high anti-Ro/SSA titers. However, ANA and anti-histone antibodies were not detected.

- Multiple scaly annular papules to few plaques over the “V” area of the neck and chest and malar erythema with minimal scaling and sparing of nasolabial folds.
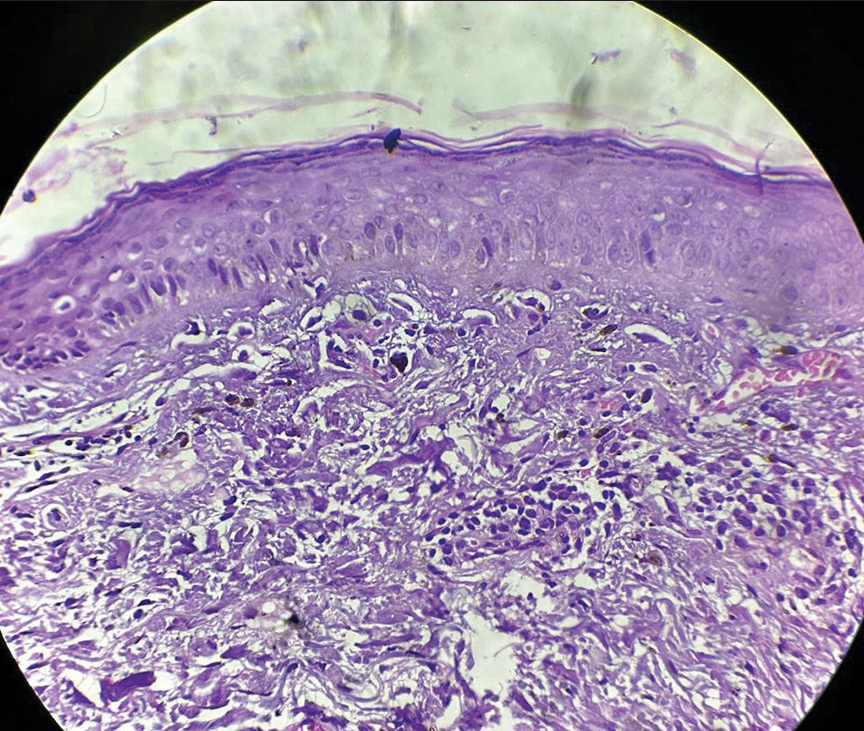
- Histopathological examination showing epidermal atrophy, basal cell degeneration, and vacuolar interface dermatitis with mild lymphocytic infiltration of the upper dermis in a perivascular pattern.
The case was discussed with the treating oncology team, and nab-paclitaxel was replaced with cisplatin. Oral antihistamine was given and she was advised to apply an emollient cream twice a day. She was re-examined after 2 weeks and showed marked improvement in terms of decreased pruritus, scaling, and erythema [Figure 3]. She was given her first “gemcitabinecisplatin” infusion after the 2 weeks of nab-paclitaxel infusion which was continued onward. There was no aggravation of existing lesions or onset of new lesions with the gemcitabinecisplatin regimen. Our differentials included drug-induced photosensitive dermatitis, de novo development of SCLE, and lichenoid drug rash. Based on the characteristic morphology of the lesions over photo-exposed sites, and the close association with a drug infusion, resolution on withdrawal of the drug, and positive anti-Ro/SSA antibodies, the final diagnosis of DISCLE secondary to Nab-paclitaxel was agreed upon.
- Clinically improving lesions of drug-induced subacute cutaneous lupus erythematosus after 4 weeks from the last dose of nab-paclitaxel.
DISCUSSION
DI-SCLE is a photosensitive subtype of cutaneous lupus erythematosus which shares cutaneous features with its idiopathic counterpart, but systemic involvement is rarely seen. It presents with symmetric, non-scarring, erythematous annular, or papulosquamous eruptions in sun-exposed areas such as face, “V” area of the neck, arms, upper back, and shoulders. The lesions heal without scarring, although, vitiligo-like hypopigmentation may be left behind.[1-4,6] DI-SCLE is known to develop secondary to more than 80 drugs including terbinafine, tumor necrosis factor-α inhibitors, anti-epileptics, anti-hypertensives, and proton-pump inhibitors[4] and constitutes a major proportions of the cases of SCLE (38%).[7]
Both nab-paclitaxel and Cremophor EL containing paclitaxel have been reported to cause DI-SCLE. Cremophor EL (vehicle agent in paclitaxel formulations) is utilized to enhance the solubility of the drug and is composed of polyoxyethylated castor oil in 50% ethanol and has also been implicated in the causation of DI-SCLE.[1,2] Gemcitabine has also been reported to be the causative factor of DI-SCLE in a few previous case reports.[7-9]
Pathogenesis of DI-SCLE remains elusive. However, taxanes such as paclitaxel have been postulated to favor apoptosis leading to a release of nucleosomes which, in turn, can trigger a local autoimmune response leading to the development of SCLE. In addition to this, increased ultraviolet light– induced apoptosis is also associated with local inflammation and increased lesional cytokine release. Both of these have been speculated to contribute to the development of typical cutaneous lesions of DI-SCLE.[2] As compared to other drugs, taxanes (paclitaxel and docetaxel) show rapid disease onset which has been highlighted in the previous case reports.[1-5]
The immunological profile of DI-SCLE may include the presence of anti-Ro/SSA and/or anti-La/SSB, together with ANA and anti-histone antibodies. However, anti-Ro/SS-A is almost equally prevalent in both DI-SCLE (80%) and idiopathic SCLE (90%).[2] Anti-histone antibodies are positive in one-third of the cases. The histopathologic findings of DI-SCLE do not differ from idiopathic SCLE, and tissue eosinophilia is not an indicator of drug-induced SCLE.[10]
In cases of drug-induced adverse reactions with combination regimen, it is difficult to point toward any one particular drug as the definitive precipitating factor. However, in our case, erythema and pruritus developed within 48 hours of the first infusion of the nab-paclitaxel. Later on, characteristic lesions of DI-SCLE appeared gradually over 8–10 days. Dermatological findings were discussed with the oncology team treating her and nab-paclitaxel was replaced with cisplatin. Symptoms started to resolve in the form of decreased scaling, erythema, and pruritus after the onset and initial progression during the first 2 weeks. The morphological transition of the lesions of DI-SCLE after the nab-paclitaxel therapy is depicted in [Figure 1] (at the 12th day) and [Figure 3] (at the 27th day). Gemcitabine has also been implicated in the DI-SCLE,[7-9] but, in our patient, cutaneous lesions of DI-SCLE were already present at the time of gemcitabine infusion (started at the end of 2nd week) and did not progress with its subsequent infusions.
Based on the typical clinical features and their close association with drug infusion, disappearance of the lesions during the drug-free period, positive antibody profile, previously published literature,[1-5] and a score of six on Naranjo drug probability scale, nab-paclitaxel was considered as the most probable cause of DI-SCLE in this patient. Given the expected resolution of skin lesions after drug withdrawal, discontinuation of the implicated medication is the cornerstone of drug-induced SCLE management. However, various therapies applied for idiopathic SCLE may also be used, including photoprotection, topical corticosteroids, and systemic antimalarials.[2]
CONCLUSION
This case is interesting as it is the second case linking the solvent (Cremophor EL) free paclitaxel to SCLE indicating that the parent drug molecule is potentially responsible for the drug reaction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-Assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- Chemotherapy in cutaneous melanoma: Is there still a role? Curr Oncol Rep. 2023;25:609-21.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced subacute cutaneous lupus erythematosus associated with nab-paclitaxel therapy. Curr Oncol. 2013;20:e484-7.
- [CrossRef] [PubMed] [Google Scholar]
- Paclitaxel-induced cutaneous lupus erythematosus in patients with serum anti-SSA/Ro antibody. J Dermatol. 2007;34:473-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous lupus erythematosus after treatment with paclitaxel and bevacizumab for metastatic breast cancer: A case report. J Med Case Rep. 2011;5:243.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced lupus erythematosus In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441889 [Last accessed on 2022 Apr 06]
- [Google Scholar]
- Subacute cutaneous lupus erythematosus induced by chemotherapy: Gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-5.
- [CrossRef] [PubMed] [Google Scholar]
- Subacute cutaneous lupus erythematosus induced by gemcitabine in 2 patients with pancreatic cancer. JAAD Case Rep. 2019;5:596-601.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine-induced subacute cutaneous lupus erythematosus: A case report. Chemotherapy. 2016;61:236-9.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents. J Dtsch Dermatol Ges. 2012;10:889-97.
- [CrossRef] [PubMed] [Google Scholar]






